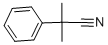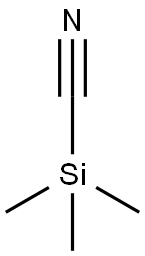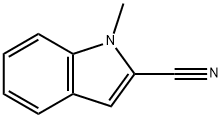
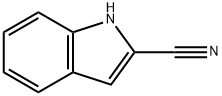
1H-Indole-2-carbonitrile,1-methyl- synthesis
- Product Name:1H-Indole-2-carbonitrile,1-methyl-
- CAS Number:60680-97-9
- Molecular formula:C10H8N2
- Molecular Weight:156.18
Yield:60680-97-9 98%
Reaction Conditions:
Stage #1: 1-methylindolewith n-butyllithium in tetrahydrofuran;hexane at 0; for 2 h;Inert atmosphere;
Stage #2: 2-methyl-2-phenylpropionitrile in tetrahydrofuran;hexane at 0 - 20; for 0.5 h;Inert atmosphere;Further stages;
Steps:
4.3. Typical procedure (2) transformation of anisole 3A into 2-methoxybenzonitrile 2A
General procedure: To a solution of anisole 3A (3.0 mmol, 324.4 mg) in THF (3.0 mL) was added n-BuLi (4.5 mmol,1.55 M in hexane, 2.87 mL) at 0 °C. The mixture was stirred for 2 h at 0 °C under an argon atmosphere. Then, pivalonitrile (9.0 mmol, 748.2 mg) in THF (2.0 mL) was added to the mixture at 0 °C and the obtained mixture was stirred for 30 min in the temperature range of 0 °C to room temperature. MeOH (2.0 mL) was added to the mixture. Then, I2 (12.0 mmol, 3045.6 mg) and K2CO3 (12.0 mmol, 1658.4 mg) were added to the mixture at room temperature, and the obtained mixture was stirred for 6 h at 70 °C. Sat. aq. Na2SO3 solution (20.0 mL) was added to the reaction mixture, and the product was extracted with AcOEt (10.0 mL x 3). The organic layer was dried over Na2SO4. After filtration and removal of the solvent, the residue was purified by silica-gel column chromatography (chloroform: n-hexane 1:1) to give 2-methoxybenzonitrile 2A (315.6 mg, 79%).
References:
Uchida, Ko;Togo, Hideo [Tetrahedron,2019,vol. 75,# 39,art. no. 130550]
603-76-9
441 suppliers
$20.00/5g
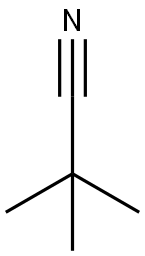
630-18-2
195 suppliers
$10.00/1g

60680-97-9
23 suppliers
inquiry

490039-74-2
0 suppliers
inquiry
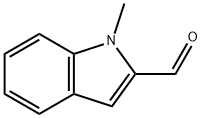
27421-51-8
84 suppliers
$45.00/50mg

60680-97-9
23 suppliers
inquiry
36193-65-4
63 suppliers
$16.00/100mg

74-88-4
360 suppliers
$15.00/10g

60680-97-9
23 suppliers
inquiry