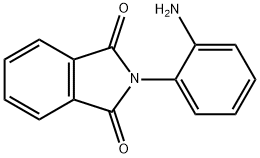
1H-Isoindole-1,3(2H)-dione, 2-(2-aMinophenyl)- synthesis
- Product Name:1H-Isoindole-1,3(2H)-dione, 2-(2-aMinophenyl)-
- CAS Number:4506-62-1
- Molecular formula:C14H10N2O2
- Molecular Weight:238.24
Yield:4506-62-1 89.2%
Reaction Conditions:
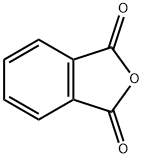
Stage #1: phthalic anhydride;1,2-diamino-benzenewith toluene-4-sulfonic acid in 5,5-dimethyl-1,3-cyclohexadiene; for 6 h;Reflux;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water at 20;
Steps:
Preparation of o-phenylenediamine protected by unilateral phthalic anhydride (a)
Dissolving 0.60 g of p-toluenesulfonic acid in xylene, first, add an equimolar amount of o-phenylenediamine to p-toluenesulfonic acid. Adding an equimolar amount of phthalic anhydride to p-toluenesulfonic acid, heating and refluxing for 6 h, After the reaction is completed, it is cooled to room temperature and the solid is filtered. washing, Drying gives o-phenylenediamine protected by p-toluenesulfonic acid and phthalic anhydride. Dissolving p-toluenesulfonic acid and phthalic anhydride-protected o-phenylenediamine into dichloromethane, the reaction was slowly carried out by slowly adding an excessive amount of a saturated aqueous solution of sodium hydrogencarbonate at room temperature. To remove p-toluenesulfonic acid, After the reaction is finished, the liquid is separated. Dried over anhydrous magnesium sulfate, the solvent was dried to give 0.74 g of o-phenylenediamine as a phthalic anhydride. The yield was 89.2%.
References:
CN108503661,2018,A Location in patent:Paragraph 0033

34442-94-9
6 suppliers
inquiry

4506-62-1
12 suppliers
$57.50/250mg
201230-82-2
1 suppliers
inquiry
610-97-9
431 suppliers
$9.00/1g
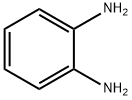
95-54-5
545 suppliers
$9.00/1g
![isoindolo[2,3-a]benzimidazol-11-one](/CAS/20180601/GIF/2717-05-7.gif)
2717-05-7
3 suppliers
inquiry

4506-62-1
12 suppliers
$57.50/250mg
![Dibenzo[b,f][1,4]diazocine-6,11-dione, 5,12-dihydro-](/CAS/20210305/GIF/4482-14-8.gif)
4482-14-8
1 suppliers
inquiry

4506-62-1
12 suppliers
$57.50/250mg