1H-Isoindole-1,3(2H)-dione, 2-methyl-5-phenoxy- synthesis
- Product Name:1H-Isoindole-1,3(2H)-dione, 2-methyl-5-phenoxy-
- CAS Number:63197-24-0
- Molecular formula:C15H11NO3
- Molecular Weight:253.25
Yield:63197-24-0 95%
Reaction Conditions:
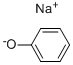
Stage #1: phenolwith sodium hydroxide in toluene; for 1 h;Reflux;
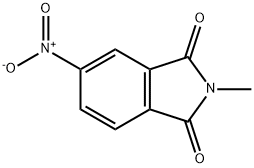
Stage #2: 4-nitro-N-methyl-phthalimide in dimethyl sulfoxide at 60; for 6 h;
Steps:
1 EXAMPLE 1. PREPARATI N OF 4-PHENOXY-N- METHYL PHTHALIMIDE OF FORMULA- VIIIB
10 N Sodium hydroxide (52.5 ml, 0.525 mole), toluene (206.2 g) and phenol (51.8 g, 0.55 mole) are mixed in a flask, heated to reflux for 60 minutes, then dehydrated by azeotropic distillation to remove water from the system. DMSO (206.2 g) is added to make a solvent exchange. The temperature is kept at 110-115°C till all of toluene are distilled away. Thereafter the reaction mixture is cooled to 60°C. 4-nitro-N-methyl phthalimide of Formula Vila (103.1 g, 0.5 mole ) is added, and continuously kept running at 60°C for 6 hours. The reaction mixture is heated and concentrated to remove most DMSO, then cooled to 20-25°C. The mixture is adjusted pH 6-7 by adding acetic acid dropwise, followed by the addition of water (35 ml). The precipitated solid is collected by filtration, rinsed with water twice (each 300 ml) and filtered. The wet product is collected and dried in the oven to give 121.3 g of 4-phenoxy-N-methyl phthalimide of Formula VHIb (yield = 95%).
References:
WO2018/140186,2018,A1 Location in patent:Paragraph 0054; 0055
63197-17-1
8 suppliers
inquiry

63197-24-0
2 suppliers
inquiry