1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID synthesis
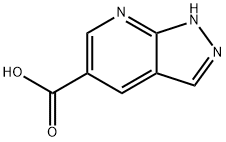
- Product Name:1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID
- CAS Number:952182-02-4
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
![1H-Pyrazolo[3,4-b]pyridine-5-carboxylic acid, 1-[(4-methoxyphenyl)methyl]-, ethyl ester](/CAS/20210111/GIF/1086423-59-7.gif)
1086423-59-7
![1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/952182-02-4.gif)
952182-02-4
Step 5. Ethyl 1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (4.4 g, 14 mmol) was dissolved in trifluoroacetic acid (TFA, 158 mL) and heated to 80 °C. The reaction mixture was stirred continuously at 80 °C for 4 hours. After completion of the reaction, the mixture was concentrated to dryness under reduced pressure. The residue was carefully poured into ice water and the pH was subsequently adjusted dropwise with 2 M aqueous sodium hydroxide (NaOH) to about 14. The precipitated solid was removed by filtration and the aqueous phase was washed with ethyl acetate. After combining the aqueous phases, the pH was adjusted to neutral (about 7) by slowly adding concentrated hydrochloric acid (HCl). The precipitate precipitated was collected by vacuum filtration and dried under vacuum to afford the target product 1H-pyrazolo[3,4-B]pyridine-5-carboxylic acid as a white solid (1 g, 80% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 14.38-13.62 (br, 1H), 9.07 (d, J = 1.6 Hz, 1H), 8.81 (d, J = 1.6 Hz, 1H), 8.32 (s, 1H). Mass spectrum (ESI+): m/z 164 [M+H]+.
![1H-Pyrazolo[3,4-b]pyridine-5-carboxylic acid, 1-[(4-methoxyphenyl)methyl]-, ethyl ester](/CAS/20210111/GIF/1086423-59-7.gif)
1086423-59-7
0 suppliers
inquiry
![1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/952182-02-4.gif)
952182-02-4
88 suppliers
$85.00/250MG
Yield: 80%
Reaction Conditions:
with trifluoroacetic acid at 80; for 4 h;
Steps:
4.5 Intermediate 4: 1H-Pyrazolo[3,4-b]pyridine-5-carboxylic acid
Step 5. Ethyl 1-(4-methoxybenzyl )-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (4.4 g, 1 4 mmol) was dissolved in TFA (1 58 mL) and heated to 80 °C. The mixture was stirred at 80 "C for 4 h, then was concentrated to dryness. The residue was poured into ice water, then aqueous NaOH solution (2 M) was added until the pH was approximately 14 The solid formed was removed by filtration, and the aqueous layer was washed with ethyl acetate. To the aqueous layer was added concentrated HCl was added until the pH was approximately 7. The resulting precipitate was collected by vacuum filtration and dried under vacuum to afford the title compound as a white solid (2. 1 g, 80%). 1H NMR (400 MHz, DMSO-d6) δ 1 4.38- 13 62 (br, 1 H), 9.07 (d, J = 1 .6 Hz, 1 H), 8.81 (d, J = 1 .6 Hz, 1 H), 8.32 (s, 1 H). MS (m/z, ESI+): 1 64 [M+H]
References:
Location in patent:Page/Page column 131; 133
![Methyl 1H-pyrazolo[3,4-b]pyridine-5-carboxylate](/CAS/GIF/1196156-42-9.gif)
1196156-42-9
79 suppliers
$110.00/50mg
![1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/952182-02-4.gif)
952182-02-4
88 suppliers
$85.00/250MG
![ethyl 4-hydroxy-1-(4-Methoxybenzyl)-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate](/CAS/20150408/GIF/227617-15-4.gif)
227617-15-4
16 suppliers
inquiry
![1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/952182-02-4.gif)
952182-02-4
88 suppliers
$85.00/250MG
![ethyl 4-chloro-1-(4-Methoxybenzyl)-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate](/CAS/20150408/GIF/227617-16-5.gif)
227617-16-5
19 suppliers
inquiry
![1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/952182-02-4.gif)
952182-02-4
88 suppliers
$85.00/250MG
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg
![1H-PYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/952182-02-4.gif)
952182-02-4
88 suppliers
$85.00/250MG