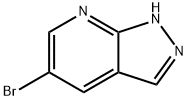
5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE synthesis
- Product Name:5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE
- CAS Number:875781-17-2
- Molecular formula:C6H4BrN3
- Molecular Weight:198.02
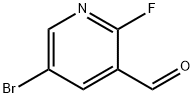
875781-15-0
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
The general procedure for the synthesis of 5-bromo-1H-pyrazolo[3,4-b]pyridine from 5-bromo-2-fluoro-3-formylpyridine was as follows: preparation of 5-bromo-1H-pyrazolo[3,4-b]pyridine (D-1-3). Compound D-1-2 (20 g, 0.1 mol) was mixed with an ethanol solution of anhydrous hydrazine (18 g, 0.56 mol) and heated to reflux overnight. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent was petroleum ether/ethyl acetate, 2:1, v/v) to confirm complete consumption of the raw material. Upon completion of the reaction, the reaction mixture was concentrated under vacuum to about 50 mL and subsequently poured into water (500 mL). The resulting mixture was filtered and the filter cake was washed sequentially with water (50mL x 3) and ether (20mL x 3). Finally, the filter cake was dried under vacuum to afford the target compound D-1-3 (9.0 g, 46% yield) as a yellow solid.

875781-15-0
183 suppliers
$6.00/1g
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg
Yield:875781-17-2 46%
Reaction Conditions:
with hydrazine in ethanolHeating / reflux;
Steps:
D-1
Preparation of 5-bromo-1H-pyrazolo[3,4-b]pyridine (D-1-3).
References:
PFIZER INC. WO2009/16460, 2009, A2 Location in patent:Page/Page column 99-100
2065-75-0
336 suppliers
$6.00/5g

1225387-53-0
68 suppliers
inquiry
![6-BROMO-PYRAZOLO[1,5-A]PYRIMIDINE](/CAS/20180808/GIF/705263-10-1.gif)
705263-10-1
169 suppliers
$9.00/250mg
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg

2065-75-0
336 suppliers
$6.00/5g

1225387-53-0
68 suppliers
inquiry
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg
1820-80-0
476 suppliers
$5.00/5g

2065-75-0
336 suppliers
$6.00/5g
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg
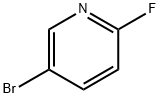
766-11-0
452 suppliers
$6.00/5g
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg