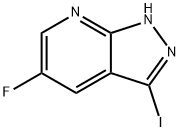
1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo- synthesis
- Product Name:1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo-
- CAS Number:1350653-23-4
- Molecular formula:C6H3FIN3
- Molecular Weight:263.01
![1H-Pyrazolo[3,4-b]pyridin-3-amine, 5-fluoro-](/CAS/GIF/1034667-22-5.gif)
1034667-22-5
102 suppliers
$22.00/100mg
![1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo-](/CAS/20150408/GIF/1350653-23-4.gif)
1350653-23-4
76 suppliers
$130.00/50mg
Yield:1350653-23-4 93%
Reaction Conditions:
Stage #1: 5-fluoro-1H-pyrazolo[3,4-b]pyridine-3-aminewith boron trifluoride diethyl ether complex;isopentyl nitrite in tetrahydrofuran at -10; for 0.5 h;
Stage #2: with sodium iodide in propan-2-one at 0 - 20; for 0.5 h;
Steps:
5 Step 5) 5-Fluoro-3-iodo-1H-pyrazolo[3,4-b]pyridine
5-Fluoro-1H-pyrazolo[3,4-b]pyridin-3-amine (5.0 g, 33 mmol) was weighed into a 500 ml flask.Tetrahydrofuran (150 mL) was added thereto, cooled to 0 ° C, and boron trifluoride diethyl ether (8.1 mL, 66 mmol) was added.Continue to cool to -10 ° C, add isoamyl nitrite (5.7 mL, 42 mmol),After stirring for 30 minutes, cold diethyl ether (100 mL) was added and filtered to give a diamine salt.Another 250 ml reaction flask was taken, and sodium iodide (6.4 g, 43 mmol) and acetone (150 mL) were added thereto.The mixture was cooled to 0 ° C, and the diazonium salt prepared above was added and allowed to react at room temperature for 30 minutes.The reaction solution was poured into ice water (300 mL) and extracted with ethyl acetate (50 mL×3).The organic phase was washed sequentially with sodium thiosulfate solution (50 mL) and brine (50 mL).Dryed over anhydrous sodium sulfate and filtered and evaporated to dryness crystals
References:
CN108690016,2018,A Location in patent:Paragraph 0379; 0380
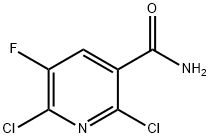
113237-20-0
188 suppliers
$5.00/250mg
![1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo-](/CAS/20150408/GIF/1350653-23-4.gif)
1350653-23-4
76 suppliers
$130.00/50mg
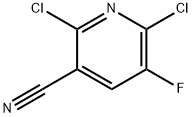
82671-02-1
223 suppliers
$10.00/5g
![1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo-](/CAS/20150408/GIF/1350653-23-4.gif)
1350653-23-4
76 suppliers
$130.00/50mg
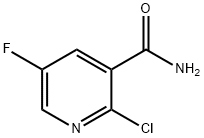
75302-64-6
52 suppliers
$240.00/100mg
![1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo-](/CAS/20150408/GIF/1350653-23-4.gif)
1350653-23-4
76 suppliers
$130.00/50mg
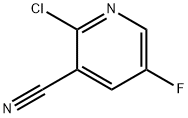
791644-48-9
141 suppliers
$16.00/100mg
![1H-Pyrazolo[3,4-b]pyridine, 5-fluoro-3-iodo-](/CAS/20150408/GIF/1350653-23-4.gif)
1350653-23-4
76 suppliers
$130.00/50mg