(1R,4R)-Sertraline HCl synthesis
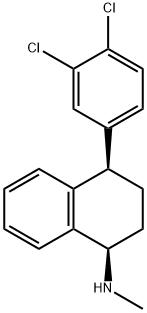
- Product Name:(1R,4R)-Sertraline HCl
- CAS Number:79617-98-4
- Molecular formula:C17H17Cl2N
- Molecular Weight:306.23
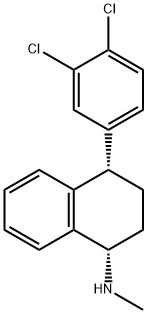
79617-96-2
159 suppliers
inquiry

79617-98-4
29 suppliers
$496.00/1mg
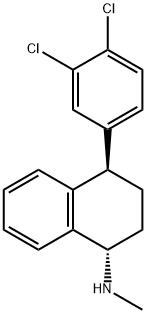
91797-60-3
1 suppliers
inquiry

79951-46-5
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: Sertralinewith potassium iodide;bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] in toluene at 80; for 1.5 h;
Stage #2: with tetra(n-butyl)ammonium hydroxide in toluene at 80; for 48 h;Product distribution / selectivity;
Steps:
3
EXAMPLE 3; (1S, 4S)-N-Methyl-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthalenamine (200 mg, 0.65 mmol) was dissolved in toluene (1.4 ml, 0.46M) under nitrogen atmosphere. Potassium iodide (108 mg, 0.65 mmol) was then added, followed by [IrCp*Cl2]2 (5 mg, 6.50 μmol) and the reaction mixture heated at 80° C. for 1.5 h. Tetrabutylammonium hydroxide (544 μl, 0.78 mmol) was then added and the reaction mixture heated at 80° C. for 48 h. The reaction mixture was then cooled down to room temperature, quenched with an aqueous solution of phosphate buffer (pH7, 5 ml), extracted with dichloromethane (5 ml), dried (Na2SO4) and concentrated under reduced pressure to give a brown oil, which was then analysed by chiral GC and the reaction shown to give a mixture of isomers in a ratio of 32.7/23.7/18.9124.7 assigned to the cis-(1S, 4S)-, cis-(1R, 4R)-, trans-(1R, 4S)- and trans-(1S,4R)-compounds respectively.
References:
US7408082,2008,B1 Location in patent:Page/Page column 14-15
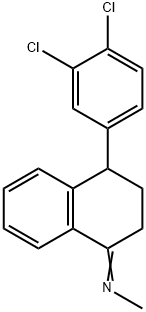
79560-20-6
117 suppliers
inquiry

79617-98-4
29 suppliers
$496.00/1mg

79951-46-5
2 suppliers
inquiry

79617-96-2
159 suppliers
inquiry

79560-20-6
117 suppliers
inquiry

79617-98-4
29 suppliers
$496.00/1mg

91797-60-3
1 suppliers
inquiry

79951-46-5
2 suppliers
inquiry

79617-96-2
159 suppliers
inquiry
6284-79-3
153 suppliers
$14.00/1g

79617-98-4
29 suppliers
$496.00/1mg
![Methanamine, N-[(4R)-4-(3,4-dichlorophenyl)-3,4-dihydro-1(2H)-naphthalenylidene]-](/CAS/20210305/GIF/312620-95-4.gif)