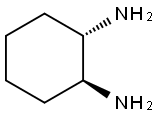
(1S,2S)-(+)-1,2-Diaminocyclohexane synthesis
- Product Name:(1S,2S)-(+)-1,2-Diaminocyclohexane
- CAS Number:21436-03-3
- Molecular formula:C6H14N2
- Molecular Weight:114.19
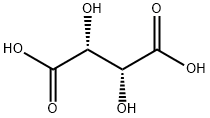
87-69-4
947 suppliers
$5.00/25g

21436-03-3
338 suppliers
$5.00/250mg
Yield:-
Reaction Conditions:
in water;acetic acid
Steps:
P.1 Resolution of Trans-1,2-diaminocyclohexane (chxn) STR8
PREPARATIVE EXAMPLE 1 Resolution of Trans-1,2-diaminocyclohexane (chxn) STR8 Racemic chxn (120 mL, 1.0 mol) was combined with water (200 mL) and L-tartaric acid (75 g, 0.5 mol). The mixture exothermed to 45° C., then was heated to 90° C. until a homogeneous solution resulted (~30 min.). The solution was cooled to 80° C., and then glacial acetic acid (50 mL, 0.873 mol) was added over 10 min., keeping the temperature below 96° C. White crystals formed during the acetic acid addition. The mixture was cooled to 0° C. and was aged at that temperature for 2 h. The crystals were collected and were washed with 0° C. water (40 mL) and 0° C. ethanol (100%, 2*50 mL). The filter cake was partially dried via suction, then was recrystallized from 1.20 L of water heated to 97° C., and then aged at ambient temperature for 18 h. The crystals were collected at ambient temperature, washed with water (40 mL) and 0° C. ethanol (40 mL). Drying the crystals at 60° C. in a vacuum oven yielded 75.7 g of 1:1 (R,R)-chxn: L-tartrate complex.
References:
Merck & Co., Inc. US5399771, 1995, A
![Benzenemethanamine, N-[(1S,2S)-2-azidocyclohexyl]-α-methyl-, (αR)-](/CAS/20211123/GIF/329321-25-7.gif)
329321-25-7
0 suppliers
inquiry

21436-03-3
338 suppliers
$5.00/250mg
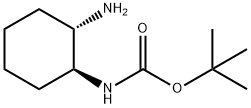
180683-64-1
133 suppliers
$21.00/100mg

21436-03-3
338 suppliers
$5.00/250mg