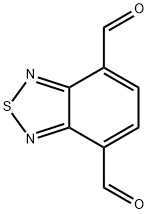
2,1,3-Benzothiadiazole-4,7-dicarboxaldehyde synthesis
- Product Name:2,1,3-Benzothiadiazole-4,7-dicarboxaldehyde
- CAS Number:5170-67-2
- Molecular formula:C8H4N2O2S
- Molecular Weight:192.19

1450737-03-7
6 suppliers
inquiry

5170-67-2
31 suppliers
inquiry
Yield:5170-67-2 34%
Reaction Conditions:
with sodium periodate in N,N-dimethyl-formamide at 150;Inert atmosphere;
Steps:
Synthesis of Benzo[c][1,2,5]thiadiazole-4,7-dicarbaldehyde(2).
Compound 1 (3.00 g, 9.30 mmol) and sodiumperiodate (NaIO4) (0.99 g, 4.8 mmol) were taken in a roundbottom flask under argon atmosphere. The above mixturewas dissolved in 30 mL of N,N-dimethylformamide (DMF)and then slowly heated to 150 °C with stirring. The progressof the reaction was monitored by thin layer chromatography(TLC) by comparison with the starting material (hexane:ethylacetate (EA), 9:1 v/v). The starting material was completelydisappeared within 60 min and two new spots were found byTLC. Solvent was completely removed by rotary evaporationand then the solid material was poured into 50 mL of water.The mixture was stirred for 30 min at RT and then extractedwith EA for three times (30 mL × 3). The combined organiclayer was washed with brine and dried over anhydrousNa2SO4. The solution was filtered and evaporated by rotaryevaporation and the crude product was purified by columnchromatography (silica gel, hexane:EA, 9:1 v/v) to affordpure compound 2 as a orange solid. Yield: (0.60 g, 34%). 1HFigure 1. Examples of benzothiadiazole (BT)-based acceptor units. NMR (300 MHz, CDCl3) δ 10.90 (s, 2 H), 8.39 (s, 2 H); 13CNMR (75 MHz, CDCl3) δ 188.7, 154.1, 131.1, 130.6; HRMS(EI+, m/z) [M+] Calcd for C8H4N2O2S 191.9993, found191.9996.
References:
Tamilavan, Vellaiappillai;Song, Myungkwan;Agneeswari, Rajalingam;Kim, Sangjun;Hyun, Myung Ho [Bulletin of the Korean Chemical Society,2014,vol. 35,# 4,p. 1098 - 1104]
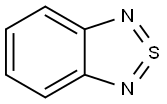
22706-22-5
14 suppliers
inquiry

5170-67-2
31 suppliers
inquiry
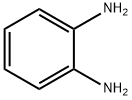
95-54-5
540 suppliers
$9.00/1g

5170-67-2
31 suppliers
inquiry