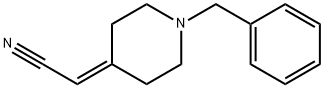
2-(1-benzylpiperidin-4-ylidene)acetonitrile synthesis
- Product Name:2-(1-benzylpiperidin-4-ylidene)acetonitrile
- CAS Number:55022-82-7
- Molecular formula:C14H16N2
- Molecular Weight:212.29
Yield:55022-82-7 95.41%
Reaction Conditions:
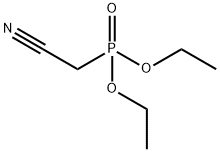
Stage #1: diethyl 1-cyanomethylphosphonatewith potassium carbonate in tetrahydrofuran at 20; for 0.583333 h;Reflux;
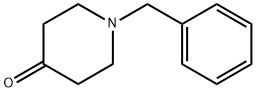
Stage #2: 1-phenylmethyl-4-piperidone in tetrahydrofuran at 70; for 16 h;
Steps:
26
A mixture of diethyl cyanomethylphosphonate (2.06 g, 1 1 .62 mmol) and anhydrous potassium carbonate (1 .6 g, 1 1 .62 mmol) in dry THF (10 mL) was stirred at room temperature for 15 min and then refluxed for 20 min. After cooling to room temperature, benzyl-piperidin-4-one (compound of Example 25; 2 g, 10.56 mmol) was added and the mixture was heated at reflux for 16 h (-70 °C). The reaction mixture was cooled to room temperature, filtered and quenched with ice water (25 mL). The resulting mixture was extracted with ethyl acetate (2 x 20 mL) and the combined organic layers were washed with water (2 x 15 mL) followed by brine (20 mL). The organic phase obtained was dried over anhydrous sodium sulphate and concentrated to obtain a crude product, which was purified by crystallisation using 2 % ethyl acetate in hexane to afford the title compound.Yield: 2.14 g (95.41 %); MS (ES+): 213 (M+1 ).
References:
WO2011/104680,2011,A1 Location in patent:Page/Page column 78

100-39-0
430 suppliers
$10.00/10g

55022-82-7
25 suppliers
inquiry
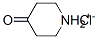
41979-39-9
0 suppliers
$15.00/10mg

55022-82-7
25 suppliers
inquiry