
2-(1-Methylethyl)-1H-pyrrolo[2,3-b]pyridine synthesis
- Product Name:2-(1-Methylethyl)-1H-pyrrolo[2,3-b]pyridine
- CAS Number:27257-18-7
- Molecular formula:C10H12N2
- Molecular Weight:160.22
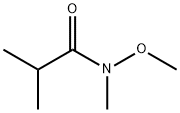
113778-69-1
79 suppliers
$29.00/1g
![2-(1-Methylethyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/27257-18-7.gif)
27257-18-7
7 suppliers
inquiry
Yield:27257-18-7 95%
Reaction Conditions:
Stage #1: tert-butyl (3-methylpyridin-2-yl)carbamatewith n-butyllithium in tetrahydrofuran at 0; for 2 h;
Stage #2: N-methoxy-N-methyl-isobutyramide in tetrahydrofuran at 0 - 20; for 1 h;
Stage #3: with hydrogenchloride;sodium hydroxidemore than 3 stages;
Steps:
To a stirred suspension of 1,1-dimethylethyl (3-methyl-2-pyridinyl)carbamate (3Og, 144mmol) in tetrahydrofuran (30OmL) at O0C under an atmosphere of nitrogen was added n-butyl-lithium (2.3M, 123mL) over 90 mins. After 30 mins at O0C λ/,2-dimethyl-/V- (methyloxy)propanamide (22.7g, 173mmol) was added. After a further 60 mins at O0C the reaction was allowed to warm to ambient temperature and was added to 5M aqueous hydrochloric acid (30OmL) and heated to 600C for 1.5 h. The organic was separated. The aqueous was basified with 10M aqueous sodium hydroxide and extracted with ethyl acetate. The combined extracts were dried (MgSO4) and concentrated in vacuo to give the title compound as an orange crystalline solid (22.04g, 95%). LCMS rt = 2.38mins, MH+= 161
References:
WO2008/34860,2008,A1 Location in patent:Page/Page column 145
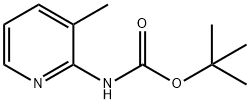
138343-75-6
126 suppliers
$8.00/1g
![2-(1-Methylethyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/27257-18-7.gif)
27257-18-7
7 suppliers
inquiry
536-78-7
207 suppliers
$25.00/5mL
100-47-0
375 suppliers
$14.14/25gm:
![3-Methyl-2-phenyl-1H-pyrrolo[2,3-b]pyridine](/CAS/20210305/GIF/139962-70-2.gif)
139962-70-2
1 suppliers
inquiry
![2-(1-Methylethyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/27257-18-7.gif)
27257-18-7
7 suppliers
inquiry
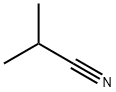
78-82-0
0 suppliers
$14.14/5gm:
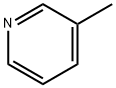
108-99-6
337 suppliers
$9.00/5g
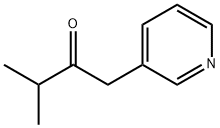
53872-97-2
6 suppliers
inquiry
![2-(1-Methylethyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/27257-18-7.gif)
27257-18-7
7 suppliers
inquiry