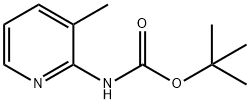
2-(BOC-AMINO)-3-METHYLPYRIDINE synthesis
- Product Name:2-(BOC-AMINO)-3-METHYLPYRIDINE
- CAS Number:138343-75-6
- Molecular formula:C11H16N2O2
- Molecular Weight:208.26
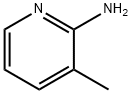
1603-40-3

24424-99-5

138343-75-6
General procedure for the synthesis of 2-(Boc-amino)-3-methylpyridine from 2-amino-3-methylpyridine and di-tert-butyl dicarbonate: di-tert-butyl dicarbonate (50.0 g) was dissolved in n-hexane (55 mL), heated to 58 °C with continuous stirring. Subsequently, a solution of ethyl acetate (20 mL) of 2-amino-3-methylpyridine (15.5 g) was added slowly and dropwise to this solution. The reaction mixture was heated and stirred at 58°C for 3 hours and then cooled to room temperature. The reaction mixture was diluted by addition of hexane (30 mL) and stirring was continued for 1.5 hours. The precipitated white solid precipitate was collected by filtration and dried to give 25.8 g of 2-(Boc-amino)-3-methylpyridine in 86% yield. Mass spectrometry (FAB) analysis showed m/z: 209 (M + H)+.

1603-40-3
489 suppliers
$6.00/25g

24424-99-5
868 suppliers
$13.50/25G

138343-75-6
126 suppliers
$8.00/1g
Yield: 90%
Reaction Conditions:
in tetrahydrofuran at 60 - 70; for 3 h;
Steps:
Synthesis of 2-(N-Tert-Butoxycarbonylamino)-3-Methylpyridine (2)
A solution of di-tert-butyl dicarbonate (12.11 g, 55.49 mmol)in tetrahydrofuran (55 mL) was stirred at 60 °C. To thissolution, 2-amino-3-methlypyridine (5 g, 46.24 mmol)was added. The mixture was heated at 70 °C for 3 h and subsequently allowed to cool to room temperature. Theprecipitate was collected by filtration and dried. The rawproduct (8.67 g) was recrystallized from dichloromethane.White crystals suitable for X-ray diffraction analysis wereobtained in a yield of 90%. m.p. 134-138 °C (Lit [16]. m.p.138-140 °C). HRMS (ESI) m/z: calcd for C11N2O2H16[M]+208.0726, found 208.0767. 1H NMR (400 MHz, CDCl3)δ8.27 (d, J = 4.8 Hz, 1H), 7.94 (s, 1H), 7.51 (d, J = 4.4 Hz,1H), 7.02 (dd, J = 7.5, 4.9 Hz, 1H), 2.28 (s, 3H), 1.50 (s,9H). 13C NMR (100 MHz, CDCl3)δ 152.74, 149.91, 145.54,139.56, 126.83, 120.47, 80.29, 28.13, 17.89.
References:
Shi, Yun-Lian;Nie, Xu-Liang;Huang, Tao;Shi, Ming-Zhu;Peng, Da-Yong;Ren, Xue-Xiang;Shi, Xu-Gen;Zhao, Ming-Yu;Li, Bao-Tong [Journal of Chemical Crystallography,2021,vol. 51,# 2,p. 155 - 160]