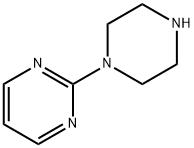
2-(1-Piperazinyl)pyrimidine synthesis
- Product Name:2-(1-Piperazinyl)pyrimidine
- CAS Number:20980-22-7
- Molecular formula:C8H12N4
- Molecular Weight:164.21
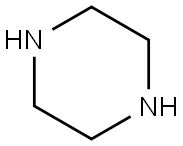
110-85-0
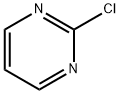
1722-12-9

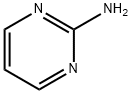
20980-22-7
Generic method: 2-chloro-4,6-disubstituted pyrimidines (17) were prepared by reaction of the diazonium salt of 4,6-disubstituted pyrimidin-2-amine (16) with concentrated hydrochloric acid and ZnCl?[35]. Compound 18 was synthesized with reference to the method of literature [32] with modifications. To a stirred solution of piperazine (45 mmol) and K?CO? (16.5 mmol) in water (20 mL) was added 2-chloro-4,6-disubstituted pyrimidine (17) (18 mmol) in batches at 50-65 °C. The reaction mixture was stirred at 60-65 °C for 1 hour and subsequently cooled to 35 °C. The solid by-product 1,4-bispyrimidinylpiperazine was removed by filtration, the filtrate was extracted three times with chloroform, the organic phase was dried with Na?SO? and concentrated under reduced pressure to give compound 18, which could be used in the subsequent reaction without further purification.18a: yellow oil, 88% yield.

110-85-0
588 suppliers
$5.00/5 g

1722-12-9
550 suppliers
$10.00/1g

20980-22-7
367 suppliers
$5.00/1g
Yield:20980-22-7 88%
Reaction Conditions:
with potassium carbonate in water at 35 - 65;
Steps:
4.2.3. Synthetic procedure of intermediates 4-(4,6-disubstitutedpyrimidin-2-yl)piperazine (18a-c)
General procedure: 2-Chloro-4,6-disubstituted-pyrimidines 17 were prepared bythe reaction of the diazoniumsalts of 4,6-disubstituted-pyrimidin-2-amines (16) with concentrated hydrochloric acid and ZnCl2 [35].Compound 18 was prepared according to literature [32], and themethod was improved. To a stirred solution of piperazine(45 mmol) and K2CO3 (16.5 mmol) in water (20 mL) was addedchloropyrimidine 17 (18 mmol) in small portions at 50e65 C. Themixture was stirred for 1 h at 60e65 C and cooled to 35 C. Theyellowsolid, 1,4-bispyrimidylpiperazine byproduct, was filtered off,and the filtrate was then extracted three times with chloroform,dried over Na2SO4, and evaporated in vacuum to give compound 18,which was used for the following reactions without further purification. 18a: yellow oil, yield 88%.
References:
Wang, Bao-Lei;Shi, Yan-Xia;Zhang, Shu-Jun;Ma, Yi;Wang, Hong-Xue;Zhang, Li-Yuan;Wei, Wei;Liu, Xing-Hai;Li, Yong-Hong;Li, Zheng-Ming;Li, Bao-Ju [European Journal of Medicinal Chemistry,2016,vol. 117,p. 167 - 178]

99931-83-6
9 suppliers
inquiry

20980-22-7
367 suppliers
$5.00/1g
780705-64-8
42 suppliers
$99.00/1 / G

20980-22-7
367 suppliers
$5.00/1g