
2(1H)-Quinoxalinone, 5-amino- synthesis
- Product Name:2(1H)-Quinoxalinone, 5-amino-
- CAS Number:1002129-56-7
- Molecular formula:C8H7N3O
- Molecular Weight:161.16
Yield:1600511-89-4 167 mg
Reaction Conditions:
with triethylamine;6-bromonicotinaldehyde in hexane;dichloromethane;acetonitrile at 0 - 20; for 3 h;
Steps:
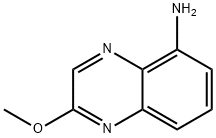
17 Synthesis of 2-Methoxyquinoxalin-5-amine
To a suspension of 5-aminoquinoxalin-2(1H)-one (440 mg, 2.73 mmol) in methanol (1 mL), DCM (8 mL) and acetonitrile (8 mL), at 0° C., was added TEA (1.14 mL, 8.19 mmol), followed by (trimethylsilyl)diazomethane (2 mL, 4.10 mmol; 2.0M in hexanes). The reaction mixture was allowed to warm to RT and stirred for additional 3 hours. The suspension was filtered, and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel chromatography, eluting with 10%-50% ethyl acetate in hexane, to provide the title compound (167 mg, 0.953 mmol) as a light-yellow powder. LC/MS (ESI+) m/z=176 (M+H)
References:
US2014/213581,2014,A1 Location in patent:Paragraph 0648; 0649

1002129-56-7
13 suppliers
inquiry
![2-[2-[(E)-2-[4-(dimethylamino)phenyl]ethenyl]-6-methylpyran-4-ylidene]propanedinitrile](/CAS/20180601/GIF/96042-30-7.gif)
96042-30-7
3 suppliers
inquiry

18107-18-1
214 suppliers
$33.00/5g
1600511-89-4
5 suppliers
inquiry
