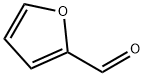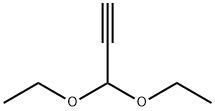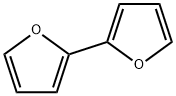
2,2'-Bifuran synthesis
- Product Name:2,2'-Bifuran
- CAS Number:5905-00-0
- Molecular formula:C8H6O2
- Molecular Weight:134.13
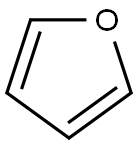
110-00-9
219 suppliers
$10.00/25g

5905-00-0
12 suppliers
inquiry
Yield: 83%
Reaction Conditions:
Stage #1:furan with n-butyllithium;N,N,N,N,-tetramethylethylenediamine in tetrahydrofuran at -78; for 0.5 h;Schlenk technique;Inert atmosphere;
Stage #2: with copper dichloride in tetrahydrofuran at 20; for 12 h;Inert atmosphere;
Steps:
4 Synthesis of [2,2'-bifuran]
In a Schlenk tube under a nitrogen stream, furan (0.5 mL, 6.88 mmol), THF (20 mL) and N, N, N ', N'-tetramethylethylenediamine (1.1 mL, 7.38 mmol) were added. Thereafter, the mixture was cooled to -78 ° C, n-BuLi (4.8 mL, 1.6M, 7.68 mmol) was added, and the mixture was stirred at -78 ° C for 30 minutes. While maintaining -78 ° C, CuCl2 (1.11 g, 8.26 mmol) was added. After slowly raising the temperature to room temperature, stirring was performed for 12 hours. After the reaction was completed, when the solution was returned to room temperature, a saturated aqueous solution of NH 4 Cl was added, and the organic layer was extracted with dichloromethane. The obtained organic layer was washed with a saturated aqueous NH 4 Cl solution and distilled water, dried over MgSO 4, filtered, and the solution was concentrated to obtain a mixture of an orange solid oil substance as an orange oil substance. (0.442 g, 3.30 mmol, 83%).
References:
Gumma University;Tachibana, Yuya;Wasano, Tatsuya;Kasuya, Kenichi JP2020/2103, 2020, A Location in patent:Paragraph 0033
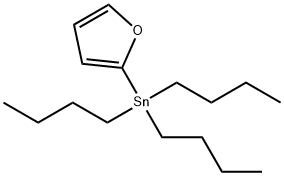
118486-94-5
124 suppliers
$27.19/5ml

5905-00-0
12 suppliers
inquiry
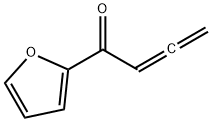
196953-18-1
0 suppliers
inquiry

5905-00-0
12 suppliers
inquiry
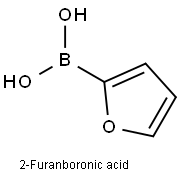
13331-23-2
345 suppliers
$13.43/250mgs:

5905-00-0
12 suppliers
inquiry