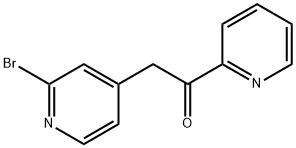
2-(2-BROMO-PYRIDIN-4-YL)-1-PYRIDIN-2-YL-ETHANONE synthesis
- Product Name:2-(2-BROMO-PYRIDIN-4-YL)-1-PYRIDIN-2-YL-ETHANONE
- CAS Number:446852-65-9
- Molecular formula:C12H9BrN2O
- Molecular Weight:277.12
Yield:446852-65-9 81%
Reaction Conditions:
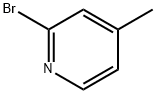
Stage #1: 2-Bromo-4-picolinewith potassium hexamethylsilazane in tetrahydrofuran;toluene at -50; for 0.5 h;Inert atmosphere;
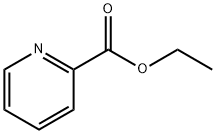
Stage #2: ethyl-2-picolinate in tetrahydrofuran;toluene at -50 - 45;Inert atmosphere;
Steps:
2-(2-Bromopyridin-4-yl)-1-(pyridine-2-yl)ethanone (11):
To a solution of 2-bromo-4-methyl pyridine (10, 5 g, 29 mmol ) in anhydrous THF (100 mL) at -50°C under a nitrogen atmosphere was added a solution of 0.5 M potassium bis-(trimethylsilyl)amide (KHMDS) in toluene (70 mL, 35 mmol) dropwise. The resultant red solution was stirred at -50°C for 30 min and then a solution of ethyl picolinate (4.83 g, 32 mmol) in anhydrous THF (50 mL) was slowly added. The mixture was warmed to room temperature and stirred for 18 h and then warmed to 45°C and stirred for 1 h. Solvent was then removed in vacuo to give a solid material which was washed with Et2O (50 mL), suspended in sat. NH4Cl (100 mL) and extracted with EtOAc (3 x 100 mL). The organic extract was dried (Na2SO4), filtered and solvent removed in vacuo to give 11 as a brown solid (6.5g, 81%). 1H NMR (300 MHz, CDCl3) δ ppm 8.72 (ddd, J = 4.8, 2.6, 1.0 Hz, 1H), 8.30 (d, J = 5.0 Hz, 1H), 8.06 (ddd, J = 7.8, 1.1, 1.1 Hz, 1H), 7.86 (ddd, J = 7.7, 7.7, 1.5 Hz, 1H), 7.52 (ddd, J = 8.1, 4.8, 1.1 Hz, 1H), 7.49 (br s, 1H), 7.24 (dd, J = 5.0, 1.5 Hz, 1H), 4.53 (s, 2H); MS (ESI+): m/z calcd. for C12H9BrN2O 276.0; obsvd. 277.1 (M + H)+.
References:
Ciayadi, Rudy;Potdar, Mahesh;Walton, Kelly L.;Harrison, Craig A.;Kelso, Geoffrey F.;Harris, Simon J.;Hearn, Milton T.W. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 18,p. 5642 - 5645] Location in patent:supporting information; experimental part