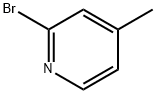
2-Bromo-4-methylpyridine synthesis
- Product Name:2-Bromo-4-methylpyridine
- CAS Number:4926-28-7
- Molecular formula:C6H6BrN
- Molecular Weight:172.02
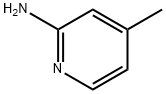
695-34-1

4926-28-7
The general procedure for the synthesis of 2-bromo-4-methylpyridine from 2-amino-4-methylpyridine was as follows: 2-amino-4-methylpyridine (120 g, 1.1 mol) was dissolved in 48% hydrobromic acid (1.5 L) and bromine (160 mL, 3.11 mol) was added slowly and dropwise at -20 °C. The reaction mixture was stirred continuously for 3 hours at -15°C to -20°C. Subsequently, aqueous sodium nitrite (NaNO2) (204 g, 2.95 mol) was added to the reaction system in batches. The reaction mixture was gradually warmed up to room temperature over a period of 3 hours. A 20% aqueous sodium hydroxide (NaOH) solution (1.2 Kg NaOH dissolved in 2 L of water) was added and the pH was adjusted to 12 while the temperature was controlled at 0 °C. The reaction mixture was extracted with ether (3 x 250 mL), the organic phase was washed sequentially with water and saturated brine and dried anhydrous. After removing the solvent under reduced pressure, the reaction was purified by fractional distillation to afford 2-bromo-4-methylpyridine (164 g, 86% yield) as a light yellow liquid.

695-34-1
537 suppliers
$5.00/10g

4926-28-7
458 suppliers
$5.00/1g
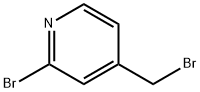
83004-14-2
73 suppliers
$33.00/100mg
Yield:-
Steps:
5.1 Step 1
Step 1 2-Bromo-4-bromomethyl pyridine Following the procedure of Adams et.al. (J. Am. Chem. Soc., 76, 3168 (1954)) 2-bromo-4-methylpyridine was obtained from 2-amino-4-methylpyridine. 1 H-NMR (CDCl3): 2.3 ppm (s, 3H); 7.05 ppm (d, 1H); 7.3 ppm (s, 1H); 8.2 ppm (s, 1H).
References:
Merck & Co., Inc. US6127390, 2000, A

695-34-1
537 suppliers
$5.00/10g

4926-28-7
458 suppliers
$5.00/1g
108-89-4
344 suppliers
$15.00/25mL

4926-28-7
458 suppliers
$5.00/1g

108-89-4
344 suppliers
$15.00/25mL
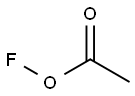
78948-09-1
2 suppliers
inquiry

4926-28-7
458 suppliers
$5.00/1g
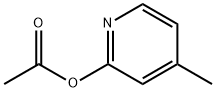
108168-80-5
1 suppliers
inquiry
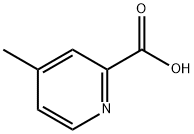
4021-08-3
142 suppliers
$11.00/250mg

4926-28-7
458 suppliers
$5.00/1g