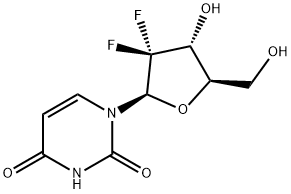
2',2'-DIFLUORO-2'-DEOXYURIDINE synthesis
- Product Name:2',2'-DIFLUORO-2'-DEOXYURIDINE
- CAS Number:114248-23-6
- Molecular formula:C9H10F2N2O5
- Molecular Weight:264.18
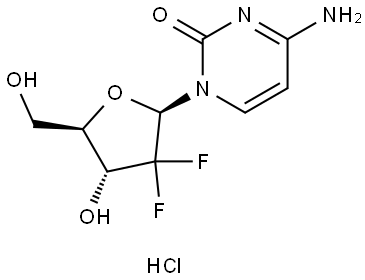
122111-03-9

114248-23-6
General procedure for the synthesis of 1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one hydrochloride from 4-amino-1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2,4(1H,3H)-dione As follows: 4-amino-1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one hydrochloride (5 g, 16.7 mmol, 1.0 eq.) was dissolved in 2N aqueous acetic acid (350 ml), sodium nitrite (16.7 g, 14.5 eq.) was added slowly and the reaction mixture The reaction mixture was allowed to stand at room temperature for 48 hours. Subsequently, the pH of the reaction mixture was adjusted to 6-7 with 2N aqueous sodium hydroxide and concentrated under reduced pressure. To the concentrated residue, a solvent mixture of methanol/ethyl acetate (1:5, 360 ml) was added and the insoluble salt was removed by filtration. This operation was repeated several times to remove the salt completely, and finally the filtrate was concentrated under reduced pressure to give 4.22 g of the target product 1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione as a light brown transparent oil in 95% yield.
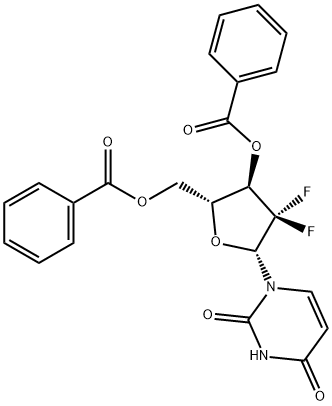
143157-27-1
29 suppliers
$70.00/10mg

114248-23-6
152 suppliers
$45.00/1mg
Yield:114248-23-6 99%
Reaction Conditions:
with ammonia in methanol at 20; for 12 h;Temperature;
Steps:
54; 134; 177 Compound 68
Compound 68-3 was dissolved in NH3/MeOH (600 mL) and stirred overnight. The solvent was concentratedto give the residue, which was purified by silica gel column chromatography (5% MeOH in DCM) to give68-4 (12 g, 99%) as a white solid
References:
US2015/105341,2015,A1 Location in patent:Paragraph 0676; 1030; 1311

122111-03-9
673 suppliers
$8.00/25mg

114248-23-6
152 suppliers
$45.00/1mg

1201895-94-4
0 suppliers
inquiry

114248-23-6
152 suppliers
$45.00/1mg
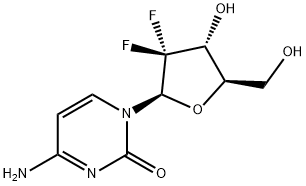
95058-81-4
551 suppliers
$6.00/100mg

114248-23-6
152 suppliers
$45.00/1mg

1445381-44-1
0 suppliers
inquiry

114248-23-6
152 suppliers
$45.00/1mg