
2,2-DIFLUORO-N-(2-HYDROXYETHYL)PROPIONAMIDE synthesis
- Product Name:2,2-DIFLUORO-N-(2-HYDROXYETHYL)PROPIONAMIDE
- CAS Number:851728-91-1
- Molecular formula:C5H9F2NO2
- Molecular Weight:153.13
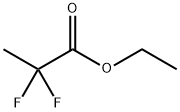
28781-85-3
212 suppliers
$15.00/1g

141-43-5
920 suppliers
$9.00/10g

851728-91-1
15 suppliers
$65.00/100mg
Yield:851728-91-1 49%
Reaction Conditions:
at 53; for 2 h;Heating / reflux;
Steps:
A 12 liter 3-necked round-bottomed flask equipped with a mechanical stirrer, thermometer, addition funnel, condenser, nitrogen inlet and drying tube was charged with 2,2-difluoro propionic acid ethyl ester from above (5557 g) and heated to reflux (approximately 53° C.). Ethanolamine (2457 g, 4023 mol) was added to the heated solution over approximately 1 hour while maintaining a gentle reflux. After the addition was complete, the mixture was refluxed for an additional hour (reaction was deemed complete by GC analysis). Ethanol was removed from the reaction mixture by vacuum distillation. The crude product was then crystallized by diluting with toluene (1:1) and followed by cooling to -20° C. After solids started to precipitate out of solution, hexane (4 ml/1 ml) was added and the mixture was allowed to stir for 2 additional hours at -20° C., followed by holding the mixture in a freezer overnight. The solids were filtered through a polypad and washed with freezer cold hexane (2* 1 liter). The isolated solids were then dried in vacuo with no heat to give 3000 g (49%) of 2,2-difluoro-N-(2-hydroxyethyl)-propionamide as a low melting solid (mp=36-38° C.).
References:
US2007/260056,2007,A1 Location in patent:Page/Page column 13-15