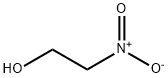
Monoethanolamine synthesis
- Product Name:Monoethanolamine
- CAS Number:141-43-5
- Molecular formula:C2H7NO
- Molecular Weight:61.08
106-57-0
238 suppliers
$11.00/5g

141-43-5
915 suppliers
$9.00/10g
Yield:141-43-5 > 99 %Spectr.
Reaction Conditions:
with Ru(H)(Cl)(PNNH(t-butyl))(CO);potassium tert-butylate;hydrogen in 1,4-dioxane at 110; under 37503.8 Torr; for 48 h;Glovebox;Inert atmosphere;Solvent;Pressure;
Steps:
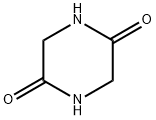
5 General Procedure for the Hydrogenation of 23 Glycine Anhydride
General procedure: In a glove box, a 100 mL Fischer-Porter tube or a 20 mL Parr apparatus was charged with catalyst (0.005 mmol), 94 KOtBu (0.006-0.012 mmol), glycine anhydride (0.5-1.0 mmol) and dioxane or THF (2 or 4 mL) under an atmosphere of purified nitrogen. The pressure equipment was taken out of the glove box, and subjected to three successive cycles of pressurization/venting with 28 H2 (3 atm), then pressurized with H2 (10-50 bar) and closed. The pressure equipment was placed behind a protective shield and the reaction mixture was heated in an oil bath at 110° C. with constant stirring for 24-48 h. After cooling to room temperature, excess H2 was vented off carefully. The unreacted glycine anhydride was filtered off washed with 10 mL of 97 hexane and dried under vacuum. To the dry solid was then added 1 mmol of 41 pyridine as an internal standard, dissolved in 42 D2O for determination of the amount of glycine anhydride by 1H NMR spectroscopy The filtrate was collected and evaporated under vacuum to give a mixture. To the mixture was added 1 mmol of pyridine as an internal standard, dissolved in D2O and analyzed by 1H NMR spectroscopy to determine the yield of 22 2-aminoethanol and the amount of glycine anhydride in solution. The total amount and the relative conversion of glycine anhydride were obtained in this way (the reason for this procedure is inaccurate determination of 2-aminoethanol in the presence of a large amount of glycine anhydride).
References:
US2017/283447,2017,A1 Location in patent:Paragraph 0281; 0325
598-42-5
92 suppliers
$61.00/1g

141-43-5
915 suppliers
$9.00/10g
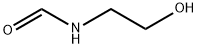
693-06-1
16 suppliers
$114.00/250mg

141-43-5
915 suppliers
$9.00/10g