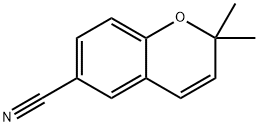
2,2-DIMETHYL-2H-CHROMENE-6-CARBONITRILE synthesis
- Product Name:2,2-DIMETHYL-2H-CHROMENE-6-CARBONITRILE
- CAS Number:33143-29-2
- Molecular formula:C12H11NO
- Molecular Weight:185.22
![4-[(1,1-DIMETHYLPROP-2-YNYL)OXY]BENZONITRILE](/CAS/GIF/33143-92-9.gif)
33143-92-9

33143-29-2
General procedure for the synthesis of 2,2-dimethyl-6-cyano-2H coumarin from 4-((2-methylbut-3-yn-2-yl)oxy)benzonitrile: 4-((2-methylbut-3-yn-2-yl)oxy)benzonitrile (1.0 g, 5.40 mmol) was dissolved in ethylene glycol (5 mL/g of substrate), and the reaction mixture was heated to 210-215 °C,. maintained for 24 hours. After completion of the reaction, the mixture was cooled to room temperature and diluted with water. The mixture was extracted with ether (2 x 25 mL), the organic layers were combined, dried over anhydrous magnesium sulfate and filtered. The solvent was removed by concentration under reduced pressure to give a bright yellow solid. Recrystallization by petroleum ether gave 2,2-dimethyl-6-cyano-2H coumarin as a yellow powder (1.0 g, quantitative yield) with a melting point of 47-48 °C (literature value: 47 °C). IR spectrum (thin film method, cm^-1): 3054, 2985, 2226, 1605, 1487, 1421, 1369, 1265, 1212, 1148, 1128, 1107, 961, 896, 828, 739, 705. ^1H NMR (400MHz, CDCl3): δ 1.45 (s, 6H). 5.70 (d, 1H, J=11.6Hz), 6.28 (d, 1H, J=11.6Hz), 6.78 (d, 1H, J=8.4Hz), 7.24 (d, 1H, J=4.0Hz), 7.37 (dd, 1H, J=8.4Hz, 4.0Hz). ^13C NMR (100 MHz, CDCl3): δ 28.4, 77.9, 103.8, 117.2, 119.3, 120.6, 121.7, 130.1, 132.2, 133.3, 156.8.
![4-[(1,1-DIMETHYLPROP-2-YNYL)OXY]BENZONITRILE](/CAS/GIF/33143-92-9.gif)
33143-92-9
46 suppliers
inquiry

33143-29-2
90 suppliers
$55.00/100mg
Yield: 100%
Reaction Conditions:
in ethylene glycol at 210 - 215; for 24 h;
Steps:
4.5. 6-Cyano-2,2-dimethylchromene 16 Method B
Phenyl propargyl ether 18 (1.0 g, 5.40 mmol) was dissolved in ethylene glycol (5 mL/g of the propargyl ether) and thereaction mixture heated to 210e215 C for 24 h. The mixture wasallowed to cool to room temperature and water added. The mixturewas extracted with Et2O (2 25 mL), the organic layers werecombined, dried over anhydrous MgSO4 and filtered. The solventswere removed under reduced pressure to give a bright yellow solid,recrystallized from light petroleumto give the title compound 16 asa yellow powder (1.0 g, quant.), mp 47e48 C [Lit.36 mp 47 C]. nmax(film)/cm1 3054, 2985, 2226, 1605, 1487, 1421, 1369, 1265, 1212,1148, 1128, 1107, 961, 896, 828, 739, 705. 1H NMR (400 MHz, CDCl3):d 1.45 (s, 6H), 5.70 (d, 1H, J 11.6 Hz), 6.28 (d, 1H, J 11.6 Hz), 6.78(d, 1H, J 8.4 Hz), 7.24 (d, 1H, J 4.0 Hz), 7.37 (dd, 1H, J 8.4 Hz,4.0 Hz); 13C NMR (100 MHz, CDCl3): d 28.4, 77.9, 103.8, 117.2, 119.3,120.6, 121.7, 130.1, 132.2, 133.3, 156.8.
References:
Bulman Page, Philip C.;Chan, Yohan;Noor Armylisas, Abu Hassan;Alahmdi, Mohammed [Tetrahedron,2016,vol. 72,# 51,p. 8406 - 8416]
1740-74-5
23 suppliers
$108.00/5g
767-00-0
581 suppliers
$6.00/25g

33143-29-2
90 suppliers
$55.00/100mg
121021-89-4
1 suppliers
inquiry

33143-29-2
90 suppliers
$55.00/100mg
108-99-6
344 suppliers
$9.00/5g

767-00-0
581 suppliers
$6.00/25g

33143-29-2
90 suppliers
$55.00/100mg

767-00-0
581 suppliers
$6.00/25g

33143-29-2
90 suppliers
$55.00/100mg