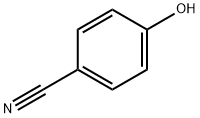
4-Cyanophenol synthesis
- Product Name:4-Cyanophenol
- CAS Number:767-00-0
- Molecular formula:C7H5NO
- Molecular Weight:119.12
1194-02-1
518 suppliers
$6.00/10g

767-00-0
572 suppliers
$6.00/25g
Yield:767-00-0 106.7 g
Reaction Conditions:
Stage #1: 4-fluorobenzonitrilewith sodium methoxide in methanol at 220; under 45004.5 Torr; for 5 h;Autoclave;Inert atmosphere;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate; pH=4;
Steps:
1 Example 1
Clean the autoclave and dry it, then add sodium methoxide methanol solution with a concentration of 30.1% to the 1000ml autoclave first(That is, the market commodity sales specification is the liquid sodium methoxide methanol solution with a content of 30% specification) 359g (2mol), then add 122g (1mol) of p-fluorobenzonitrile with a mass fraction of 99%, and the feeding ends.After the above-mentioned feeding is finished, nitrogen is flushed into the autoclave, and the air in the autoclave is replaced with nitrogen, the purpose is to remove the air in the autoclave, prevent side reactions from increasing due to the presence of air in the autoclave, and reduce the utilization rate of raw materials;And after ensuring that no air in the autoclave is all nitrogen, turn on the heating device, heat the autoclave to 220°C, and the pressure in the autoclave to 6.0Mpa,Under this condition, the raw materials react in the autoclave, and the reaction time of the raw materials in the autoclave is 5 hours, and the reaction time is calculated artificially, and the reaction ends after the time reaches 5 hours. After finishing the above reaction, stop the heating control program on the interface of the autoclave control box, pump a cooling circulating refrigerant into the coil of the autoclave, rapidly reduce the temperature of the autoclave, and finally control the temperature in the autoclave to cool down to 10°C; After cooling to 10°C, the pressure gauge drops to 0.3MPa, the pressure is released from the autoclave, a clean gas collection container is prepared, the gas output channel or valve of the autoclave is opened, and the prepared clean gas collection container is connected to the pressure. The gas output channel or valve of the kettle is connected to collect gas;After the gas collection operation is completed, the collected gas is cooled to -30 ° C to obtain a liquefied liquid, and the liquefied liquid is detected by gas chromatography,The detection result is that the content of dimethyl ether in the -30 liquefied liquid is 96.5%, the content of methanol is 2.2%, and the yield of dimethyl ether in this reaction is 95.6%;Fig. 3 is the gas chromatography spectrogram of the dimethyl ether synthesized in this Example 1.Compared with the method of methanol dehydration and adding a catalyst, the reaction of the present invention has a high yield for preparing dimethyl ether, no catalyst is added in the middle, or the reaction raw materials or reaction products play a catalytic role;The reaction pressure for preparing dimethyl ether in the previous patent documents is generally less than 3 Mpa, and the present invention breaks the conventional pressure limit and adopts a reaction pressure greater than or equal to 3 Mpa,Preferably 3.5Mpa6Mpa, the results found that the reaction pressure in this example is 6.0Mpa, and the prepared dimethyl ether yield is 95.6%. After the gas dimethyl ether was collected, 181 g of methanol was recovered by distillation under reduced pressure, and 300 g of water was added to dissolve the distillation substrate. After filtering the insolubles, acidification was performed with a concentration of 36% hydrochloric acid solution with a mass of 105 g, and the filtrate after filtration was adjusted. The PH value reaches PH=4, and the white product that separates out is p-hydroxybenzonitrile. After drying, it is detected that the p-hydroxybenzonitrile content is 99.0% (liquid chromatography, external standard method), and the weighing mass is 106.7 g, and the p-hydroxy The yield of benzonitrile was 88.7%.
References:
CN114853577,2022,A Location in patent:Paragraph 0015; 0028-0034
623-03-0
474 suppliers
$6.00/25g

767-00-0
572 suppliers
$6.00/25g
99-76-3
809 suppliers
$5.00/25g

767-00-0
572 suppliers
$6.00/25g
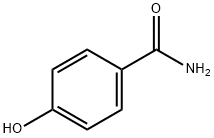
619-57-8
213 suppliers
$5.00/1g

767-00-0
572 suppliers
$6.00/25g

124-38-9
133 suppliers
$214.00/14L
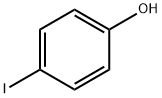
540-38-5
342 suppliers
$10.00/1g

767-00-0
572 suppliers
$6.00/25g