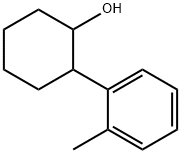
2-(2-methylphenyl)cyclohexan-1-ol synthesis
- Product Name:2-(2-methylphenyl)cyclohexan-1-ol
- CAS Number:523-25-1
- Molecular formula:C13H18O
- Molecular Weight:190.28
Yield:523-25-1 57.1%
Reaction Conditions:
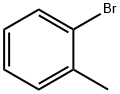
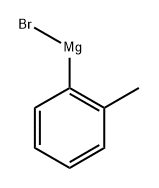
Stage #1: 2-methylphenyl bromidewith n-butyllithium in tetrahydrofuran at -90; for 1 h;Inert atmosphere;
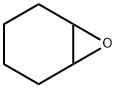
Stage #2: cyclohexane-1,2-epoxidewith boron trifluoride diethyl etherate in tetrahydrofuran at -90; for 1.5 h;Inert atmosphere;
Steps:
1.a Step a: Preparation of 1-a
2-Bromotoluene (13 g, 76 mmol) was dissolved in dry THF (120 mL), protected by Ar, liquid nitrogen ethanol was cooled to -90 ° C, n-BuLi (31.92 mL, 79.8 mmol) was added dropwise, and the drop was completed in 30 minutes.After stirring at -90 ° C for 30 min, epoxycyclohexane (8.49 mL, 83.6 mmol) and boron trifluoride ether solution (10.55 mL, 83.6 mmol) were added dropwise, and stirring was continued in liquid nitrogen ethanol for 1.5 hours. The reaction was monitored by TLC (EA: PE = 1: 5). After the reaction was completed, the reaction was quenched with saturated NH4Cl solution (100 mL), diluted with H2O (150 ml), and extracted with ethyl acetate (150 mL × 3). It was washed with saturated NaCl solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was subjected to rotary evaporation to remove low boiling solvents, and column chromatography (EA: PE = 1: 30-1: 5),8.25 g of a yellow oil was obtained in a yield of 57.1%.
References:
CN110343050,2019,A Location in patent:Paragraph 0149-0153