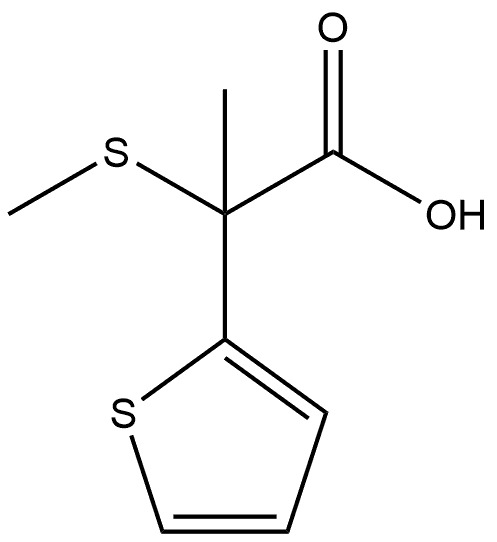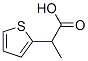
2-(2-thienyl)propionic acid synthesis
- Product Name:2-(2-thienyl)propionic acid
- CAS Number:54955-39-4
- Molecular formula:C7H8O2S
- Molecular Weight:156.2
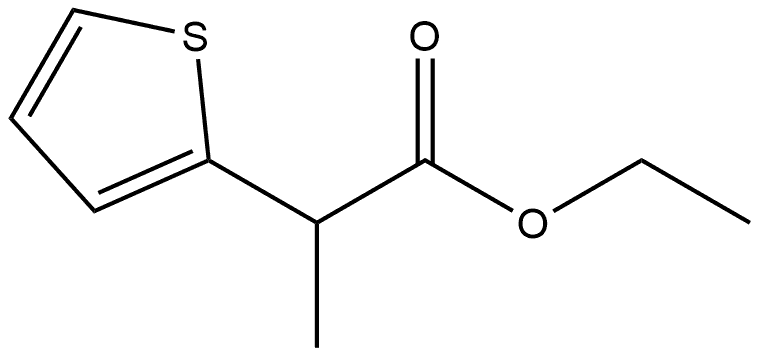
63476-16-4
0 suppliers
inquiry

54955-39-4
22 suppliers
inquiry
Yield:54955-39-4 100%
Reaction Conditions:
with lithium hydroxide monohydrate;water in methanol at 20; for 3 h;
Steps:
4 2-(Thiophen-2 I)propanoic acid
To a solution of ethyl 2-(thiophen-2-yl)propanoate (5.9 g, 32.39 mmol) in methanol (40 mL) and water (15 mL) was added lithium hydroxide monohydrate (2.0 g, 48.4 mmol) and the reaction mixture stirred at RT for 3 H. The reaction mixture was concentrated in vacuo to approximately volume and the residue extracted with EtOAc. The organic fractions were discarded and the aqueous layer was acidified to ~ pH 3 with IN HC1 and extracted with EtOAc. The combined organic fractions were washed with brine, dried (MgS04) and concentrated in vacuo to yield the title compound as a yellow oil (5.0 g, 100%). NMR (400 MHz, d6-DMSO) δ 7.26 (1H, dd, J - 5.2, 1.6 Hz), 6.89 (1H, dd, J = 5.1, 3.5 Hz), 6.87-6.80 (1H, m), 3.74 (1H, q, J = 7.0 Hz), 1.35 (3H, d, J = 7.0 Hz).
References:
WO2019/87146,2019,A1 Location in patent:Page/Page column 28
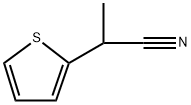
88701-59-1
4 suppliers
inquiry

54955-39-4
22 suppliers
inquiry
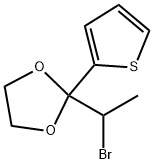
1374221-92-7
0 suppliers
inquiry

54955-39-4
22 suppliers
inquiry