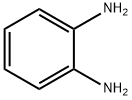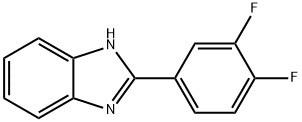
2-(3,4-Difluorophenyl)benzimidazole, 95% synthesis
- Product Name:2-(3,4-Difluorophenyl)benzimidazole, 95%
- CAS Number:1081112-48-2
- Molecular formula:C13H8F2N2
- Molecular Weight:230.21
Yield:1081112-48-2 91%
Reaction Conditions:
in neat (no solvent) at 135; for 24 h;Green chemistry;
Steps:
General procedure
General procedure: Reactions were performed in a magnetically stirred round bottomed flask fitted with acondenser and placed in a temperature controlled oil bath. 1,2-Diamine (2 mmol)was added to alcohol (3 mmol) and the reaction mixture was allowed to stir at 135°C in an open (air) atmosphere. After disappearance of the diamine (reaction was monitored by TLC)or after the appropriate time, the reaction mixture was cooled to roomtemperature. The crude residue was further purified by column chromatography using silica gel (100-200 mesh) to afford pure products. All the products wereidentified on the basis of NMR and mass spectral data
References:
Marri, Mahender Reddy;Peraka, Swamy;Macharla, Arun Kumar;Mameda, Naresh;Kodumuri, Srujana;Nama, Narender [Tetrahedron Letters,2014,vol. 55,# 48,p. 6520 - 6525] Location in patent:supporting information