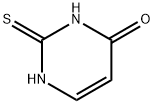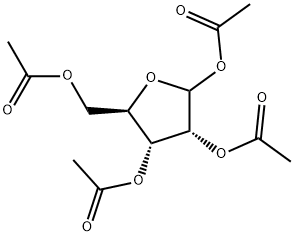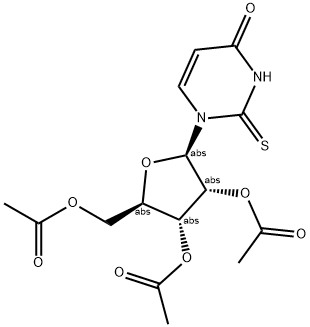
2',3',5'-Tri-O-acetyl-2-thiouridine synthesis
- Product Name:2',3',5'-Tri-O-acetyl-2-thiouridine
- CAS Number:28542-31-6
- Molecular formula:C15H18N2O8S
- Molecular Weight:386.377
Yield:28542-31-6 89%
Reaction Conditions:
Stage #1: 2-thiouracilwith ammonium sulfate;chloro-trimethyl-silane;1,1,1,3,3,3-hexamethyl-disilazane at 126; for 18 h;
Stage #2: 1,2,3,5-tetra-O-acetyl-D-ribofuranosewith tin(IV) chloride in 1,2-dichloro-ethane at 20; for 1 h;
Steps:
37; 40; 48
Synthesis of Compound 1 39. To a solution of compound 1 14 (24.0 g, 1 87.2 mmol) in xamethyldisilazane (960 ml_) were added trimethyl chlorosilane (21 .60 ml_, 1 69.70 mmol) and the alytic amount of ammonium sulfate (1 .0 g, 75 mmol). The clear reaction mixture was stirred at 126 for 1 8 h. The reaction mixture became clear, and concentrated under reduced pressure to dryness not more than 45 °C. A solution of 1 ,2,3,5-tetra-O-acetyl-D-robofuranose (66 g, 207.60 mmol) in 1 ,2-dichloroethane (240 ml_) was added to the reaction mixture, followed by addition of tin achloride (28.80 ml_, 249.6 mmol). The reaction mixture was stirred at room temperature for 1 h. on comp dium bica and washed xtracted with dichloromethane. The combined organic phase was dried over anhydrous Na2S04. e drying agent was filtered off, and the filtrate was concentrated under reduced pressure. The de product was purified by flash chromatography on a silica gel column using ethyl acetate - roleum ether (1 :2 to 1 :1 ) resulting in compound 139 (65.0 g, 1 68.2 mmol) in 89 % yield.
References:
WO2015/196128,2015,A2 Location in patent:Page/Page column 620; 625; 627; 628
13035-61-5
598 suppliers
$5.00/25g

28542-31-6
13 suppliers
inquiry