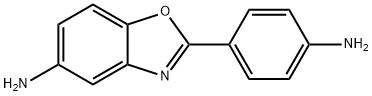
2-(3-AMINO-PHENYL)-BENZOOXAZOL-5-YLAMINE synthesis
- Product Name:2-(3-AMINO-PHENYL)-BENZOOXAZOL-5-YLAMINE
- CAS Number:13676-47-6
- Molecular formula:C13H11N3O
- Molecular Weight:225.25
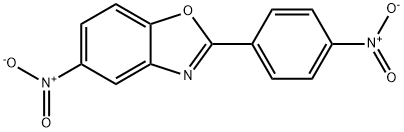
1037-39-4

13676-47-6
General procedure for the synthesis of 2-(4-aminophenyl)-5-aminobenzo[d]oxazole from 2-(4-nitrophenyl)-5-nitrobenzo[d]oxazole: 2-(4-nitrophenyl)-5-nitrobenzo[d]oxazole (3.22 g, 0.01 mol) was added to a 100 mL single-necked flask followed by the addition of 50 mL of water at room temperature. The pH of the reaction system was adjusted with phosphoric acid to 1. Then, sodium thiosulfate solid (8.5 g, 0.06 mol) was added and the reaction system was warmed up to 100 °C for 16 h. The reaction was completed by adding a solid sodium thiosulfate (8.5 g, 0.06 mol). After completion of the reaction, it was cooled to room temperature and filtered. To the filtrate, 25% by weight of aqueous sodium carbonate was added and neutralized to pH 7. At this point, the product 2-(4-aminophenyl)-5-aminobenzo[d]oxazole was precipitated from the reaction system, and after filtration, several washes, and drying, 2.14 g of the target product was obtained (95% yield, HPLC purity >99.9%).

1037-39-4
1 suppliers
inquiry

13676-47-6
145 suppliers
$23.00/250mg
Yield:13676-47-6 95%
Reaction Conditions:
with phosphoric acid;sodium sulfite in water at 100; pH=< 1; for 16 h;
Steps:
2 n the compound having the structure represented by the formula (I), R is a structure represented by the formula (2), and 2-(4-nitrophenyl)-5-nitrobenzoxazole is obtained.
At room temperature,Add 2-(4-nitrophenyl)-5-nitrobenzoxazole (3.22 g, to a 100 mL ml single-necked flask, 0.01 mol) and 50 mL of water,Adding phosphoric acid,Adjust the pH below 1.JoinSodium thiosulfitesolidbody(8.5g, 0.06mol),Warming up to 100 ° C,Continuous reaction for 16 h.After going to room temperature,filter,The filtrate was neutralized to a pH of 7 by adding a 25 wt% aqueous solution of sodium carbonate.The product precipitates from the reaction system,filter,Washed many times,dry,2.14 g of the product 2-(4-aminophenyl)-5-aminobenzoxazole were obtained (yield 95%, purity (HPLC) >99.9%).
References:
CN108558770,2018,A Location in patent:Paragraph 0082; 0083; 0084
137-09-7
161 suppliers
$32.47/25G
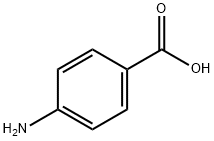
150-13-0
957 suppliers
$5.00/25g

13676-47-6
145 suppliers
$23.00/250mg

150-13-0
957 suppliers
$5.00/25g
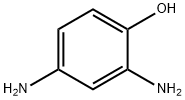
95-86-3
47 suppliers
$84.00/1g

13676-47-6
145 suppliers
$23.00/250mg
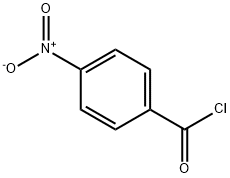
122-04-3
38 suppliers
$15.00/1g

13676-47-6
145 suppliers
$23.00/250mg