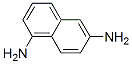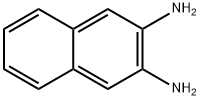
2,3-diaminonaphthalene synthesis
- Product Name: 2,3-diaminonaphthalene
- CAS Number:771-97-1
- Molecular formula:C10H10N2
- Molecular Weight:158.2
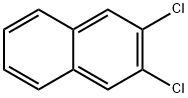
2050-75-1

771-97-1
General procedure for the synthesis of 2,3-diaminonaphthalene from 2,3-dichloronaphthalene: Dissolve 2,3-dichloronaphthalene (2 g) in anhydrous ethanol. Ammonium chloride (1.5 g) was added and diluted. Subsequently, a catalytic amount of hydrochloric acid and an excess of concentrated aqueous ammonia solution were added to the reaction system and the reaction was refluxed on a water bath for 6 hours until the reaction solution showed a persistent dark green color. After completion of the reaction, the reaction solution was cooled and placed in a deep freezer overnight. Green crystals were obtained by filtration, dried and recrystallized with methanol. Dark green crystals were obtained; 75% yield; thin layer chromatography Rf values of 0.89 and 0.82; melting point 150 °C; IR spectra (νmax, KBr, cm-1): 758, 854, 1473 (aromatic C-N stretching vibration), 1500 (aromatic C=C stretching vibration), 1681 (N-H bending vibration), 3049 (N-H stretching vibration); 1H NMR (CDCl3, 500 MHz): δ 7.45-7.46 (dd, J=3.5-4 Hz, 2H, Ar-H), 7.12-7.14 (dd, J=3-3.5 Hz, 2H, Ar-H), 7.09 (s, 2H, Ar-H), 7.05 (s, 4H, Ar-NH2).

2050-75-1
20 suppliers
inquiry

771-97-1
280 suppliers
$7.00/100mg
Yield:771-97-1 75%
Reaction Conditions:
with hydrogenchloride;ammonia;ammonium chloride in ethanol for 6 h;Reflux;
Steps:
Synthesis of 2,3-Diaminonaphthalene (2)
Compound 1 (2 gm) was dissolved in absolute ethanol. Ammonium chloride (1.5 gm),dil. HCl (in catalytic amount) and excess of strong ammonium solution were added to it and reuxed for 6 h on water bath till appearing of permanent dark greenish colour. After completion of reaction, solution was cooled and kept in deep freezer for overnight. Greenish crystals were fltered, dried and recrystallized with methanol. Dark greenish crystals;Yield 75%; Rf 0.89, Rf 0.82; mp 150 °C; IR (νmax,KBr, cm-1): 758, 854, 1473 (aromatic =C-N), 1500(aromatic C=C), 1681 (N-H), 3049 (N-H); 1H-NMR(CDCl3 500 MHz): 7.45-7.46 (dd, J=3.5-4 Hz, 2H,Ar-H), 7.12-7.14 (dd, J=3-3.5 Hz, 2H, Ar-H), 7.09 (s,Ar-H, 2H), 7.05 (s, Ar-NH2,4H).
References:
Kumar, Shiv;Kumar, Nitin;Drabu, Sushma [Oriental Journal of Chemistry,2017,vol. 33,# 2,p. 821 - 828]
92-44-4
376 suppliers
$9.00/1g

771-97-1
280 suppliers
$7.00/100mg
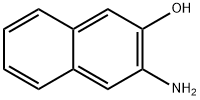
5417-63-0
125 suppliers
$10.00/100mg

771-97-1
280 suppliers
$7.00/100mg
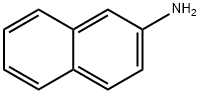
91-59-8
210 suppliers
$54.00/100mg

771-97-1
280 suppliers
$7.00/100mg