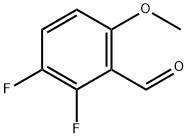
2,3-DIFLUORO-6-METHOXYBENZALDEHYDE synthesis
- Product Name:2,3-DIFLUORO-6-METHOXYBENZALDEHYDE
- CAS Number:187543-87-9
- Molecular formula:C8H6F2O2
- Molecular Weight:172.13

68-12-2
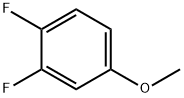
115144-40-6

187543-87-9
a) General procedure for the synthesis of 2,3-difluoro-6-methoxybenzaldehyde: A 2 M solution of diisopropylaminolithium THF/n-heptane (171 mL, 341 mmol) was diluted with dry THF (250 mL) under nitrogen protection and cooled to -75°C. A solution of 3,4-difluoroanisole (46.8 g, 325 mmol) in anhydrous THF (100 mL) was added slowly dropwise and stirred for 1 hour keeping the temperature at -75 °C. Subsequently, dry N,N-dimethylformamide (27.6 mL, 358 mmol) was added slowly and stirring was continued at -70 °C for 10 min. After the reaction was completed, acetic acid (30 mL) and water (400 mL) were added and warmed to 10 °C. The reaction mixture was extracted with ether (2 x 300 mL). The organic phases were combined and washed sequentially with water (250 mL), 0.2N aqueous hydrochloric acid (400 mL) and brine (2 x 250 mL), dried with anhydrous magnesium sulfate, and the solvent was concentrated under reduced pressure to give a red to orange oil, which was allowed to stand and crystallized. Purification by recrystallization from ether/petroleum ether (40-60 °C) afforded the target product 2,3-difluoro-6-methoxybenzaldehyde (53.0 g, 95% yield).1H NMR (300 MHz, CDCl3) δ: 10.40 (1H, s, CHO), 7.37 (1H, q, ArH), 6.71 (1H, m, ArH), 3.93 ( 3H, s, OCH3).
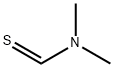
758-16-7
106 suppliers
$21.21/5gm:

115144-40-6
225 suppliers
$9.00/5g

187543-87-9
162 suppliers
$25.00/250mg
Yield:187543-87-9 95%
Reaction Conditions:
Stage #1: 1,2-difluoro-4-methoxy-benzenewith lithium diisopropyl amide in tetrahydrofuran at -75; for 1 h;
Stage #2: N,N-dimethylthioformamide in tetrahydrofuran;water;acetic acid at -70 - 10; for 0.166667 h;
Steps:
2,3-Difluoro-6-methoxybenzaldehyde
2, 3-DIFLUORO-6-METHOXYBENZALDEHYDE A solution of lithium diisopropylamide, 2M in THF/N-HEPTANE (171 mL, 341 mmol) was further diluted with dry THF (250 ML) and cooled under nitrogen TO-75C. 3,4- Difluoroanisole (46.8 g, 325 mmol) in dry THF (100 ML) was added dropwise and the mixture stirred at-75C for LH. Dry N, N dimethylformamide (27.6 ML, 358 mmol) was added dropwise and the mixture stirred for 10 mins AT-70C. Acetic acid (30 ML) and water (400 ML) were added, warming the temperature to 10C. Extracted into diethyl ether (2 x 300 mL). Combined extracts were washed with water (250 mL), aqueous hydrochloric acid (0.2 N, 400 mL) and brine (2 x 250 mL), dried (MgSO4) and the solvent evaporated in vacuo to give a RED/ORANGE oil which crystallised. Purification was by recrystallisation from diethyl ETHER/PETROLEUM ether 40-60 to give (53. 0g, 95%) of solid; ON (300 MHz, CDC13) 10.40 (1H, s, CHO), 7.37 (1H, q, ArH), 6.71 (1H, m, ArH), and 3.93 (3H, s OCH3).
References:
WO2004/43903,2004,A1 Location in patent:Page 40 - 41
4394-85-8
327 suppliers
$6.00/25g

115144-40-6
225 suppliers
$9.00/5g

187543-87-9
162 suppliers
$25.00/250mg

115144-40-6
225 suppliers
$9.00/5g

187543-87-9
162 suppliers
$25.00/250mg
2713-33-9
318 suppliers
$9.00/5g

187543-87-9
162 suppliers
$25.00/250mg