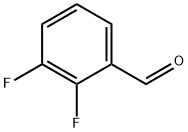
2,3-Difluorobenzaldehyde synthesis
- Product Name:2,3-Difluorobenzaldehyde
- CAS Number:2646-91-5
- Molecular formula:C7H4F2O
- Molecular Weight:142.1
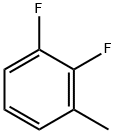
3828-49-7

2646-91-5
The general steps for the synthesis of 2,3-difluorobenzaldehyde from 2,3-difluorotoluene are as follows: (1) Preparation of the device: connect the tubular reactor as shown in Fig. 2, and choose a combination of pipes with (3a + 3c) DC channels and inclined square pancake-type variable diameter flat pipes. Determine the pipe diameter and volume according to the experimental needs. (2) Solution preparation: 6.06 g of cobalt acetate and 6.06 g of sodium molybdate were dissolved in 200 mL of acetic acid containing 200 mg of 2,3-difluorotoluene to form a mixed solution, ensuring that n (cobalt acetate): n (2,3-difluorotoluene) = 0.015:1. Meanwhile, 6.06 g of sodium bromide was dissolved in 35% H2O2 to prepare a H2O2-acetic acid solution, adjusting n (sodium bromide): n(2,3-difluorotoluene) = 0.015: 1. (3) Reaction process: 2,3-difluorotoluene-acetic acid solution and H2O2-acetic acid solution were injected into a continuous heat exchanger tubular reactor through a constant-flow pump at a flow rate of 5.56 ml/min and 11.11 ml/min, respectively, to ensure that n(H2O2): n(2,3-difluorotoluene) = 2:1. The reaction was carried out in a microchannel reactor with a set reaction temperature of 120 °C and a residence time of 1500 s. (4) Post-treatment: after completion of the reaction, the outlet material was cooled and the reaction was quenched with dichloromethane. The conversion of 2,3-difluorotoluene was determined to be 65.1% and the yield of 2,3-difluorobenzaldehyde was 48.2% by GC analysis.

3828-49-7
258 suppliers
$6.00/1g

2646-91-5
299 suppliers
$5.00/250mg
Yield:2646-91-5 48.2%
Reaction Conditions:
with sodium molybdate;dihydrogen peroxide;cobalt(II) acetate;acetic acid;sodium bromide at 120;Temperature;
Steps:
9
(1) Apparatus: The connection type of the tubular reactor is determined according to Fig. 2, and the pipe type is: (3a + 3c) DC type channel + inclined square cake type variable diameter flat pipe,Pipeline diameter and volume according to the flow rate and reaction time to determine,Heat transfer medium for the heat transfer oil.(2) 6.06 g of cobalt acetate and 6.06 g of sodium molybdate were respectively dissolved200 mg of 2,3-difluorotoluene and 200 ml of acetic acid to form a mixed solution at which time n (cobalt acetate): n (2,3-difluorotoluene) = 0.015: 1, 6.06 g of sodium bromide was dissolved in35% H2O2 to form H2O2-acetic acid solution, then n (sodium bromide):N (2,3-difluorotoluene) = 0.015: 1,2,3-difluorotoluene-acetic acid solution with andH2O2-acetic acid solution at 5.56 ml / min and 11.11 ml / min, respectivelyOf the flow rate through the constant current pump into the continuous heat exchanger tube reactor,At this time n (H2O2): n (2,3-difluorotoluene) = 2: 1, using Figure 2 microchannel reactor,Control reaction temperature 120 , residence time 1500s. Export material 0 cooling,The reaction was quenched with difluoromethane. After GC analysis,The conversion of 2,3-difluorotoluene was 65.1% and the yield of 2,3-difluorobenzaldehyde was 48.2%.
References:
Changzhou University;Liu Jianwu;Jiang Xin;Zhang Yue;Yan Shenghu;Shen Jiefa;Ma Xiaoming;Chen Daixiang;Gu Shunlin;Ni Fengchao;Li Yanfei;Wang Qiuhong;Chen Mingzhu CN106748682, 2017, A Location in patent:Paragraph 0052-0054