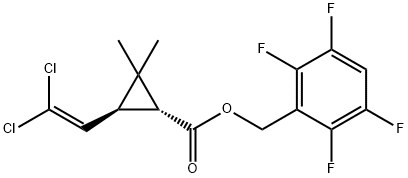
Transfluthrin synthesis
- Product Name:Transfluthrin
- CAS Number:118712-89-3
- Molecular formula:C15H12Cl2F4O2
- Molecular Weight:371.15
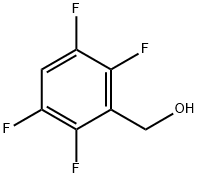
61914-47-4
2 suppliers
inquiry
4084-38-2
280 suppliers
$9.00/5g

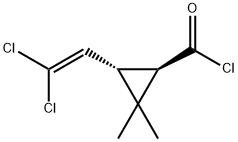
118712-89-3
181 suppliers
$49.00/250mg
Yield:118712-89-3 97.6%
Reaction Conditions:
with pyridine in toluene at 5 - 15; for 3 h;
Steps:
5 Example 1 Synthesis of (f?S)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1 f?,3/:?)-2,2-dimethyl - (0119) 3-(2-methylprop-1 -enyl) cyclopropanecarboxylate (D-trans-allethrin)
General procedure: In 1 liter three necked bottom flask with a stirrer, 51 .0 g (0.33 moles) of (f?S)-3- allyl-2-methyl-4-oxocyclopent-2-enyl-1 -ol with a purity of 99% (%w/w) were mixed with 180 ml of toluene and cooled to 5°C under nitrogen atmosphere. The solution was added with 34.5 g (0.43 moles) of pyridine and added slowly and under stirring with 65.0 g (0.34 moles) of (1 R,3R)-2,2-dimethyl-3-(2-methylprop- 1 -enyl) cyclopropane carboxylic acid chloride with a purity of 98.0% (% w/w), keeping the temperature at 5°C-10°C . After the addition, the reaction was heated to 15°-20°C under stirring for further 3 hours. (0121) 4.1 g (0.05 moles) of 1 ,3-diaminopropane were added and the reaction was maintained under stirring for further Vz hour. The reaction was then added with an acid aqueous solution (0.36 moles of HCI 33%(%w/w) in 135 ml of water), stirred and the organic phase separated off. The organic phase was then washed with water, separated and washed again with 65 ml of an aqueous solution containing 8% (% w/v) of sodium carbonate. The organic phase was separated and evaporated u.v at 40°C/ 0.2 kPa. (0122) 98.5 g of a crude product with a purity of 96.8% (% w/w) was obtained (yield 95%) whose NMR analysis is in accordance with the structure. (0123) The amount of chrysanthemic anhydride was less than 0.02% (%w/w) by GC analysis as previously reported. (0124) 1H-NMR (300 MHz, CDCIs): 1 .15 (s, 2.25H), 1 .1 6 (s, 0.75H), 1 .27 (s, 2.25H), 1 .29 (s, 0.75H), 1 .41 (d, 0.25H, 3J = 5.5 Hz), 1 .43 (d, 0.75H, 3J = 5.5 Hz), 1 .70 - 1 .75 (m, 6H), 2.02 (s, 2.25H), 2.04 (s, 0.75H), 2.10 (dd, 1 H, 3J = 7.7 Hz, 3J = 5.2 Hz), 2.24 (dd, 0.75H, 2J = 18.7 Hz, 3J = 2.0 Hz), 2.31 (dd, 0.25H, 2J = 18.7 Hz, 3J = 2.0 Hz), 2.86 (dd, 0.25H, 2J = 18.7 Hz, 3J = 7.3 Hz), 2.88 (dd, 0.75H, 2J = 18.7 Hz, 3J = 6.3 Hz), 2.99 (d, 2H, 3J = 6.4 Hz), 4.92 (dm, 1 H, 3J = 7.5 Hz), 5.02 (m, 2H), 5.69 (m, 1 H), 5.77 (m, 1 H). (0125) 13C-NMR (75 MHz, CDCIs): 13.75 (CH3), 13.87 (CH3), 18.34 (CH3), 20.24 (CH3), 20.37 (CH3), 21 .95 (CH3), 22.00 (CH3), 25.39 (CH3), 26.97 (CH2), 28.93 (Cq), 32.81 (CH), 33.01 (CH), 34.37 (CH), 34.42 (CH), 41 .46 (CH2), 41 .88 (CH2), 72.47 (CH), 72.77 (CH), 1 15.78 (CH2), 120.60 (CH), 120.67 (CH), 133.41 (CH), 135.70 (Cq), 141 .15 (Cq), 141 .22 (Cq), 1 65.76(Cq), 1 65.86 (Cq), 172.12 (Cq), 203.59 (Cq), 203.65 (Cq).
References:
ENDURA S.P.A.;ZAMBONIN, Giuliano;LOLLI, Simona;GUERRINI, Alberto;BORZATTA, Valerio WO2018/50213, 2018, A1 Location in patent:Page/Page column 10; 11; 14