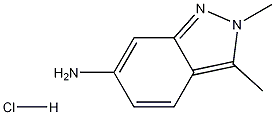
2,3-dimethyl-2H-indazol-6-amine hydrochloride synthesis
- Product Name:2,3-dimethyl-2H-indazol-6-amine hydrochloride
- CAS Number:635702-60-2
- Molecular formula:C9H12ClN3
- Molecular Weight:197.6647
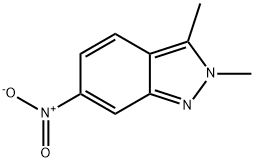
444731-73-1

635702-60-2
General procedure for the synthesis of 2,3-dimethyl-6-amino-2H-indazole hydrochloride from 2,3-dimethyl-6-nitroindazole: 2,3-dimethyl-6-nitro-2H-indazole (1.13 g) was dissolved in 2-methoxyethyl ether (12 mL) under stirring at 0°C, and then 4.48 g of stannous chloride in 8.9 mL of concentrated hydrochloric acid was added drop by drop over a period of 5 minutes solution. After the dropwise addition was completed, the ice bath was removed and the reaction mixture continued to be stirred for 30 minutes. Subsequently, about 40 mL of diethyl ether was added to the reaction solution to induce precipitation of the product. The precipitate was separated by filtration and washed with diethyl ether to afford the yellow solid product 2,3-dimethyl-6-amino-2H-indazole hydrochloride (1.1 g, 95% yield). The structure of the product was confirmed by 1H NMR (300 MHz, DMSO-d6) and mass spectrometry (ES+): 1H NMR δ 7.77 (d, J=8.9Hz, 1H), 7.18 (s, 1H), 7.88 (m, 1H), 4.04 (s, 3H), 2.61 (s, 3H); MS (ES+, m/z) 162 (M+H).

444731-73-1
209 suppliers
$25.00/100mg

635702-60-2
246 suppliers
$10.00/1g
Yield:635702-60-2 96%
Reaction Conditions:
with hydrogenchloride;tin(ll) chloride in diethylene glycol dimethyl ether;water at 0; for 0.583333 h;
Steps:
2.1 Intermediate Example 2; Preparation of 2,3-dimethyl-6-amino-2H-indazole; Procedure 1
To a stirred solution of 2, [3-DIMETHYL-6-NITRO-2H-INDAZOLE] (1.13 g) in 2- methoxyethyl ether (12 [ML),] at [0] [C,] was added a solution of 4.48 g of tin [(LL)] chloride in 8.9 ml of concentrated HCI dropwise over 5 min. After the addition was complete, the ice bath was removed and the solution was allowed to stir for an additional 30 min. Approximately 40 ml of diethyl ether was added to reaction, resulting in precipitate formation. The resulting precipitate was isolated by filtration and washed with diethyl ether, and afforded a yellow solid (1.1 g, 95 [%),] the HCI salt 2,3- [DIMETHYL-2H-INDAZOL-6-AMINE.'H] NMR (300 MHz, [DMSO-D6)] 8 7. [77] (d, [J =] 8.9 [HZ,] [1 H),] 7.18 (s, [1 H),] 7.88 (m, [1 H),] 4.04 (s, 3H), 2.61 (s, 3H). MS [(ES+,] m/z) 162 (M+H).
References:
WO2003/106416,2003,A2 Location in patent:Page 42-43
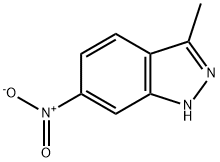
6494-19-5
260 suppliers
$19.00/1g

635702-60-2
246 suppliers
$10.00/1g

635702-59-9
0 suppliers
inquiry

635702-60-2
246 suppliers
$10.00/1g
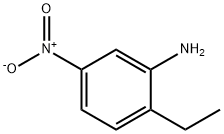
20191-74-6
130 suppliers
$6.00/250mg

635702-60-2
246 suppliers
$10.00/1g