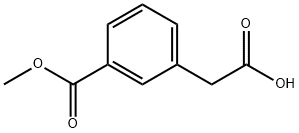
2-(3-(methoxycarbonyl)phenyl)acetic acid synthesis
- Product Name:2-(3-(methoxycarbonyl)phenyl)acetic acid
- CAS Number:52787-19-6
- Molecular formula:C10H10O4
- Molecular Weight:194.18
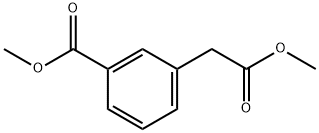
52787-20-9

52787-19-6
Methyl 3-(methoxycarbonyl)benzoate 76 (500 mg, 2.40 mmol) was used as a raw material and dissolved in methanol (10 mL). To this solution was added a solution of K2CO3 (700 mg, 5.07 mmol) in water (10 mL) and the reaction was stirred overnight at room temperature. Upon completion of the reaction, the reaction mixture was concentrated and dissolved in water (20 mL). Subsequently, the unreacted diester 76 was extracted with water and EtOAc, and the aqueous phase was acidified with 1N HCl and then extracted with EtOAc (30 mL x 2). The organic layers were combined, dried with anhydrous sodium sulfate and concentrated in vacuum. The crude product was purified by silica gel column chromatography (eluent: hexane/EtOAc, 5:1) to afford the target product 2-(3-(methoxycarbonyl)phenyl)acetic acid 77 (508 mg, 90% yield). The product was detected by TLC (Rf = 0.36), 1H NMR (400 MHz, methanol-d4) δ 7.97 (d, J = 1.7 Hz, 1H), 7.91 (dt, J = 7.8,1.4 Hz, 1H), 7.57-7.49 (m, 1H), 7.43 (t, J = 7.6 Hz, 1H), 3.91 (s, 3H), and 3.67 (s, 2H); mass spectral analysis showed [M + H]+ = 195.14 (APCI+).

52787-20-9
10 suppliers
inquiry

52787-19-6
52 suppliers
$70.00/50mg
Yield: 90%
Reaction Conditions:
Stage #1:3-methoxycarbonylphenylacetic acid methyl ester with potassium carbonate in methanol;water at 20; for 12 h;
Stage #2: with hydrogenchloride in water
Steps:
14 2- (3- (methoxycarbonyl)phenyl) acetic acid (77)
A solution of K2C03 (700 mg, 5.07 mmol) in H20 (10 mL) was added to a solution of methyl 3- (2-methoxy-2-oxoethyl) benzoate 76, (500 mg, 2.40 mmol) in methanol (10 mL) and stirred overnight at room temperature. Reaction mixture was concentrated and dissolved in water (20 mL) . Aqueous later was extracted with EtOAc to the unreacted diester 76, acidified with IN HC1, and extracted with EtOAc (30 mL x 2) . Organic layer was dried (anhydrous sodium sulfate) and concentrated in vacuo. The crude product was chromatographed on silica gel (Hexanes/EtOAc, 5:1) to yield target compound 77. Yield 508 mg, 90%. Rf = 0.36, H 1H NMR (400 MHz, Methanol-d4) δ 7.97 (d, J = 1.7 Hz, 1H) , 7.91 (dt, J = 7.8, 1.4 Hz, 1H) , 7.57 - 7.49 (m, 1H) , 7.43 (t, J = 7.6 Hz, 1H) , 3.91 (s, 3H) , 3.67 (s, 2H) ; [M+H]+ = 195.14 (APCI+) .
References:
Location in patent:Page/Page column 63; 65
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

52787-19-6
52 suppliers
$70.00/50mg

124-38-9
134 suppliers
$175.00/23402
34040-63-6
85 suppliers
$45.00/100mg

52787-19-6
52 suppliers
$70.00/50mg
153599-45-2
26 suppliers
inquiry

52787-19-6
52 suppliers
$70.00/50mg
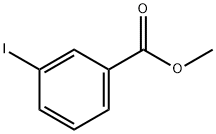
618-91-7
139 suppliers
$11.00/5g
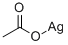
563-63-3
283 suppliers
$7.00/1g
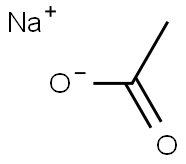
127-09-3
1177 suppliers
$8.00/1 ea

52787-19-6
52 suppliers
$70.00/50mg