2-(3-METHYLPYRIDIN-2-YL)ACETONITRILE synthesis
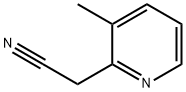
- Product Name:2-(3-METHYLPYRIDIN-2-YL)ACETONITRILE
- CAS Number:38203-11-1
- Molecular formula:C8H8N2
- Molecular Weight:132.16
Yield:38203-11-1 100%
Reaction Conditions:
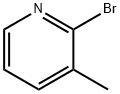
with n-butyllithium in tetrahydrofuran;hexanes at -78; for 2.08333 h;
Steps:
129.b
2-(3-Methylpyridine-2-yl)acetonitrile: To a solution of n-BuLi (2.5 N in hexanes, 7.92 mL, 19.8 mmol) at -78° C. under N2 was added dry THF (75 mL), followed immediately by a solution of dry MeCN (1.15 mL, 21.78 mmol) in anhydrous THF (30 mL) over a 5-min period. The resulting reaction mixture was stirred continuously at -78° C. for 1 h. Then 2-bromo-3-methylpyridine (516 mg, 3 mmol) was added. The resulting reaction mixture was stirred at -78° C. for 1 h, then warmed to room temperature, and quenched with water. The organic solvent was evaporated in vacuo, dissolved in CH2Cl2. The organic layer was washed with brine, dried (MgSO4), concentrated, purified via column chromatography (20% EtOAc in hexanes) to afford the product quantitatively: m/e=133 [M+1].
References:
US2005/84506,2005,A1 Location in patent:Page/Page column 79