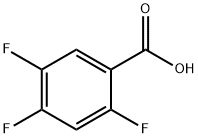
2,4,5-Trifluorobenzoic acid synthesis
- Product Name:2,4,5-Trifluorobenzoic acid
- CAS Number:446-17-3
- Molecular formula:C7H3F3O2
- Molecular Weight:176.09
3811-75-4
37 suppliers
inquiry

446-17-3
411 suppliers
$6.00/5g
Yield:446-17-3 87.8%
Reaction Conditions:
in water;ethyl acetate;
Steps:
EXAMPLE 1 Perforation of 2,4,5-Trifluorobenzoic Acid by the Decarboxylation of 3,4,6-Trifluorophthalic Acid in N-Methylpyrrolidone (NMP)
EXAMPLE 1 Perforation of 2,4,5-Trifluorobenzoic Acid by the Decarboxylation of 3,4,6-Trifluorophthalic Acid in N-Methylpyrrolidone (NMP) A 250 mL single-neck flask equipped with a condenser and a magnetic stirrer was charged with 30.00 g of 3,4,6-trifluorophthalic acid, and 200 mL of NMP. The reaction mixture was then heated with stirring for 18 hr. at a bath temperature of 140° C., followed by 55 hr. at a bath temperature of 150° C. The flask was then allowed to cool to room temperature and the contents were poured into 500 mL of water, and extracted with 4*200 mL of ethyl acetate. The combined organic extracts were washed with water (2*200 mL), dried over magnesium sulfate, filtered, and the solvent removed on a rotary evaporator followed by drying at the pump overnight. The crude product (24.25 g) was then dissolved in 25 mL of ethyl acetate and introduced at the top of a silica column (4.5 cm ID*56 cm, flushed with hexane). The product was then eluted with 90:10 hexane:ethyl acetate under a slight nitrogen pressure, and the fractions (500 mL each) were monitored by gas chromatography. The fractions containing the product were combined, the solvent removed on a rotary evaporator, and then dried at the pump overnight to give 2,4,5-trifluorobenzoic acid as a light yellow solid (21.06 g, 98.97% pure, 87.8% yield). Recrystallization of the product from toluene (50 mL, ca. 60° C.) gave a white product (71.1% overall yield) mp 101°-102° C.
References:
US5233085,1993,A
129322-83-4
169 suppliers
$13.00/1g

446-17-3
411 suppliers
$6.00/5g
327-52-6
330 suppliers
$6.00/5g

446-17-3
411 suppliers
$6.00/5g
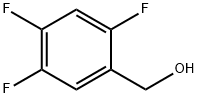
144284-25-3
160 suppliers
$8.00/1g

446-17-3
411 suppliers
$6.00/5g
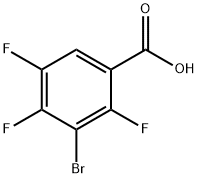
104222-42-6
79 suppliers
$37.00/1g

446-17-3
411 suppliers
$6.00/5g