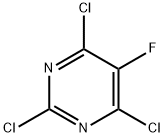
2,4,6-TRICHLORO-5-FLUOROPYRIMIDINE synthesis
- Product Name:2,4,6-TRICHLORO-5-FLUOROPYRIMIDINE
- CAS Number:6693-08-9
- Molecular formula:C4Cl3FN2
- Molecular Weight:201.41
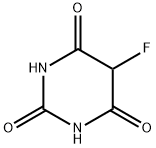
767-80-6

6693-08-9
Part B: Synthesis of 2,4,6-trichloro-5-fluoropyrimidine. Under stirring conditions (note the exothermic reaction), finely powdered 5-fluoro-6-hydroxy-2,4(1H,3H)-pyrimidinedione (74 g, 0.507 mol) was added to phosphorus oxychloride (POCl3, 232 mL, 2.5 mol) in a batch over a period of 30 min. After the addition was completed, the reaction mixture was maintained at 60 °C and N,N-dimethylaniline (65 mL) was slowly added dropwise via syringe. Upon completion of the dropwise addition, the reaction system was heated to 100-110 °C (internal temperature) and the reaction was continued until the reaction was determined to be complete by TLC or other appropriate method, which typically took 4-8 hours. Upon completion of the reaction, the mixture is cooled to room temperature, followed by careful removal of most of the remaining phosphorous trichloride by vacuum distillation at 80-90°C (note: some of the product may be distilled out with POCl3). The residue was slowly poured into ice water (~1 L) and stirred for 30 min. Extraction was carried out with ether (1 x 400 mL, 2 x 150 mL), the organic phases were combined and washed sequentially with water and saturated saline, and dried over anhydrous magnesium sulfate. The desiccant was removed by filtration, and the ether was removed by atmospheric pressure distillation to obtain the crude product. The crude product was purified by distillation under reduced pressure to afford the target product 2,4,6-trichloro-5-fluoropyrimidine (28.8 g, 28% yield) as a low melting point white crystalline solid (boiling point 80-85 °C, 12 mmHg).LCMS analysis: (M + H)+: not detected.

767-80-6
53 suppliers
$119.00/1g

6693-08-9
103 suppliers
$26.00/100mg
Yield:6693-08-9 55%
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-aniline at 110; for 8 h;Inert atmosphere;
Steps:
1.2 Step 2: Synthesis of Intermediate Int-2
The intermediates Int-1 (95g, 650mmol), POCl3 (425ml, 6552mmol) and 83ml of N,N-dimethylaniline were slowly added dropwise to the round bottom flask under nitrogen conditions, and then stirred under reflux at 110° C. for 8 hours. After the reaction is over, cool it to room temperature and slowly pour the reactant into excess ice water (beware of severe exothermic reaction). After extraction with ethyl acetate and drying with MgSO4, filter. The filtrate was distilled under reduced pressure to obtain 72 g (55%) of an intermediate Int-2.
References:
Samsung SDI Co., Ltd;Kim Chang-u;Kim Hyeong-seon;Ryu Dong-wan;Shin Chang-ju;Lee Seung-jae;Lee Mi-jin;Lee Hyeon-gyu;Jeong Seong-hyeon;Jeong Ho-guk KR2020/86131, 2020, A Location in patent:Paragraph 0311-0312; 0316-0317

767-80-6
53 suppliers
$119.00/1g

6693-08-9
103 suppliers
$26.00/100mg

767-80-6
53 suppliers
$119.00/1g

6693-08-9
103 suppliers
$26.00/100mg
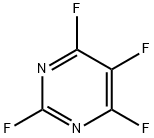
767-79-3
27 suppliers
$127.00/500mg
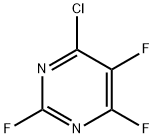
96819-50-0
0 suppliers
inquiry
96819-51-1
2 suppliers
inquiry

6693-08-9
103 suppliers
$26.00/100mg

96819-51-1
2 suppliers
inquiry

6693-08-9
103 suppliers
$26.00/100mg