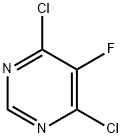
4,6-Dichloro-5-fluoropyrimidine synthesis
- Product Name:4,6-Dichloro-5-fluoropyrimidine
- CAS Number:213265-83-9
- Molecular formula:C4HCl2FN2
- Molecular Weight:166.97
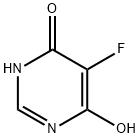
106615-61-6

213265-83-9
General procedure for the synthesis of 4,6-dichloro-5-fluoropyrimidine from 5-fluoro-4,6-dihydroxypyrimidine (Method B): phosphoryl chloride (0.593 L, 6.376 mol) was slowly added to a 2 L three-necked round-bottomed flask containing 5-fluoropyrimidine-4,6-diol (276.459 g, 2.125 mol) to form a slurry at room temperature. N,N-dimethylaniline (81 mL, 0.638 mol) was slowly added to this slurry through the addition funnel and the reaction was exothermic. The reaction mixture was continued to be stirred at 110°C for 6 hours. Upon completion of the reaction, the mixture was cooled to room temperature and slowly poured into a 2 L mixture consisting of brine and ice under stirring. The aqueous layer (showing red color) was extracted with dichloromethane (2 x 2 L). The organic layers were combined and washed sequentially with cold 6 N hydrochloric acid (2 × 1 L, showing honey brown color) and saturated sodium bicarbonate solution (1 L). The organic layer was dried with anhydrous sodium sulfate, filtered through glass fiber paper under vacuum, and concentrated under reduced pressure (without heating) to afford 4,6-dichloro-5-fluoropyrimidine (CAS No. 213265-83-9, 347.9 g, 2.084 mol, 98% yield) as an amber colored oil. Nuclear magnetic resonance (NMR) analysis showed traces of dichloromethane in the product. 4,6-Dichloro-5-fluoropyrimidine (420 g, 2.515 mol) was subjected to vacuum distillation, and the fraction with a boiling point of 35°C (pressure 1 torr) was collected to give a colorless oily substance (332.574 g, 1.992 mol, 79% yield). The product solidified at -78°C and remelted to liquid at room temperature. Distillation conditions: oil bath temperature 100°C; product boiling point 35°C; pressure 1 Torr. 1H NMR (400 MHz, DMSO-d6) δ 8.8 (s, 1H).

106615-61-6
137 suppliers
$18.00/250mg

213265-83-9
200 suppliers
$12.00/1g
Yield:213265-83-9 98%
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-aniline at 110; for 6 h;Product distribution / selectivity;
Steps:
1.2
Method BTo a 2 L three necked round bottom flask containing 5-fluoropyrimidine-4,6-diol(276.459 g, 2.125 mol) was added at room temperature slowly phosphoryl chloride (0.593 L, 6.376 mol) to form a slurry. To this slurry was added N,N-dimethylaniline (81 mL, 0.638 mol) very slowly using an addition funnel (exothermic) and the reaction was continued for 6 h at 110 °C. After 6 h, the reaction mixture was cooled to room temperature and slowly added into brine and ice (2 L) with stirring. The aqueous layer (red) was extracted with DCM (2 x 2 L). The combined organic layers were washed with cold 6 Ν HC1 (honey brown) (2 x 1 L) and washed with sat NaHC03 (1 L). The organic layer was dried (Na2S04), filtered by vacuum filtration through a glass fiber paper and solvent was removed under reduced pressure (no heat) to give 4,6-dichloro-5-fluoropyrimidine (CASNo.213265-83-9, 347.9 g, 2.084 mol, 98% yield) as an amber oil. NMR showed the oil contained traces of DCM. 4,6-Dichloro-5-fluoropyrimidine (420 g, 2.515 mol) was distilled by vacuum distillation to give 4,6-dichloro-5-fluoropyrimidine (332.574 g, 1.992 mol, 79% yield) as a colorless oil. The product solidified in the flask at -78 °C and melts when brought to room temperature. Conditions for distillation: Oil Bath: 100 °C; Product boiling temp: 35 °C; Pressure: 1 Torr. lU NMR (400 MHz, DMSO-i¾) δ ppm 8.8 (s, 1H).
References:
ARENA PHARMACEUTICALS, INC.;JONES, Robert M.;LEHMANN, Juerg;CHEN, Weichao;EDWARDS, Jeffrey;MARQUEZ, Glen;MORGAN, Michael E.;SADEQUE, Abu J.M. WO2012/40279, 2012, A1 Location in patent:Page/Page column 86
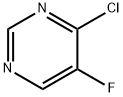
347418-42-2
119 suppliers
$37.00/1g

213265-83-9
200 suppliers
$12.00/1g