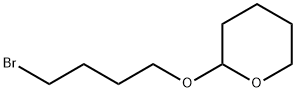
2-(4-Bromobutoxy)tetrahydro-2H-pyran synthesis
- Product Name:2-(4-Bromobutoxy)tetrahydro-2H-pyran
- CAS Number:31608-22-7
- Molecular formula:C9H17BrO2
- Molecular Weight:237.13
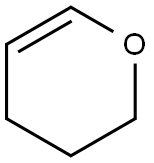
110-87-2

33036-62-3

31608-22-7
General procedure for the synthesis of 2-(4-bromobutoxy)tetrahydro-2H-pyran from 3,4-dihydro-2H-pyran and 4-bromo-1-butanol: 3,4-dihydro-2H-pyran (8.0 g, 95.36 mmol) was slowly added to a solution of 4-bromo-1-butanol (12.0 g, 79.47 mmol) in dichloromethane (150 mL) at 0 °C , followed by the addition of p-toluenesulfonic acid (20 mg) as a catalyst. After 1 hour of reaction, the reaction was carefully quenched with saturated NaHCO3 solution (5 mL), followed by washing the organic layer with water (100 mL) and brine (70 mL) sequentially. The organic layer was concentrated in vacuum to give the crude product. The residue was purified by silica gel column chromatography using 2% ethyl acetate/hexane as eluent to afford 2-(4-bromobutoxy)tetrahydro-2H-pyran (16.57 g, 88% yield) as a colorless oil. Thin layer chromatography (TLC) analytical conditions: 10% ethyl acetate/hexane, Rf value 0.50. 1H NMR (CDCl3, 300MHz) δ 4.58 (t, J = 2.5Hz, 1H), 3.90-3.72 (m, 2H), 3.38-3.50 (m, 4H), 1.92-2.04 (m, 2H), 1.65-1.80 ( m, 4H), 1.50-1.60 (m, 4H).

51326-51-3
18 suppliers
$205.00/2g

31608-22-7
67 suppliers
$75.00/1g
Yield:31608-22-7 80%
Reaction Conditions:
with 1H-imidazole;bromine;triphenylphosphine in toluene at 0 - 20; for 3 h;Inert atmosphere;Large scale;
Steps:
4 Example 3 Preparation of SLP-7b
In a 10-liter reactor, 640 g of SLP-6, 5 liters of toluene, 1.0 kg of triphenylphosphine, and 320 g of imidazole were added and the mixture was stirred until dissolution was complete. Under a nitrogen atmosphere, after the temperature was reduced to 0 degrees, 570 g of liquid was added in portions. The bromine was controlled so that the reaction temperature did not exceed 10 degrees; after the addition was complete, the reaction was warmed to room temperature for 3 hours until the end of the reaction.Add 3 liters of water and stir and let stand.The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to give the crude product; the crude product was purified by column chromatography on ethyl acetate/petroleum ether to give 697 g of SLP-7b (X=Br) as a pale yellow oil.Yield: 80%.H NMR (400MHz, CDCl3): δ 4.57 (m, 1H), 3.93-3.78 (m, 2H), 3.52-3.40 (m, 2H), 3.22 (t, 2H), 1.93-1.50 (m, 10H)ESI/MS+(m/z):237
References:
Shanghai Aikangrui Pharmaceutical Technology Co., Ltd.;Fan Linfeng;Zhu Yangwei;Qiu Aiyun;Lv Bojie;Xu Zhonghui;Zhang Changxuan CN106316967, 2017, A Location in patent:Paragraph 0063; 0079; 0080; 0081; 0082; 0083

110-87-2
544 suppliers
$10.00/1g

33036-62-3
240 suppliers
$45.00/1g

31608-22-7
67 suppliers
$75.00/1g
109-99-9
1102 suppliers
$9.00/5ml

110-87-2
544 suppliers
$10.00/1g

31608-22-7
67 suppliers
$75.00/1g

110-87-2
544 suppliers
$10.00/1g

31608-22-7
67 suppliers
$75.00/1g
1927-68-0
16 suppliers
inquiry

31608-22-7
67 suppliers
$75.00/1g