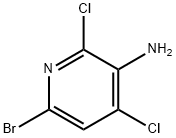
2,4-DICHLORO-3-AMINO-6-BROMOPYRIDINE synthesis
- Product Name:2,4-DICHLORO-3-AMINO-6-BROMOPYRIDINE
- CAS Number:237435-16-4
- Molecular formula:C5H3BrCl2N2
- Molecular Weight:241.9
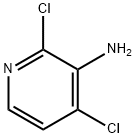
173772-63-9

237435-16-4
(d) Synthesis of 3-amino-6-bromo-2,4-dichloropyridine. 3-Amino-2,4-dichloropyridine (500 mg, 3.1 mmol) was dissolved in N,N-dimethylformamide (DMF, 16 mL) and cooled to 0 °C in an ice-water bath. Subsequently, a solution of N-bromosuccinimide (660 mg, 3.7 mmol) in DMF (7 mL) was slowly added dropwise. After 15 min of reaction, the reaction mixture was poured into water and extracted with ethyl acetate (EtOAc, 2×). The organic phases were combined, washed sequentially with water and saturated saline, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure to give a red residue. The residue was dissolved in a mixed solvent of ethyl acetate/hexane with a volume ratio of 1:1, filtered through a silica gel short column, and concentrated again under reduced pressure to give 0.68 g of the title compound in colorless crystalline form (90% yield). 1H NMR (DMSO-d6, 500 MHz): δ 6.10 (s, 2H), 7.69 (s, 1H). Mass spectrum (MS) m/z: 243 ([M+H]+, 81Br), 241 ([M+H]+, 79Br).
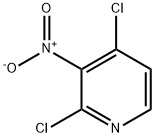
5975-12-2
232 suppliers
$10.00/1g

237435-16-4
55 suppliers
$50.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 90 percent / SnCl2*2H2O; conc. aq. HCl / diethyl ether / 20 °C
2: 90 percent / N-bromosuccinimide / dimethylformamide / 0.25 h / 0 °C
References:
Norman;Chen;Chen;Fotsch;Hale;Han;Hurt;Jenkins;Kincaid;Liu;Lu;Moreno;Santora;Sonnenberg;Karbon [Journal of Medicinal Chemistry,2000,vol. 43,# 22,p. 4288 - 4312]

173772-63-9
132 suppliers
$11.00/250mg

237435-16-4
55 suppliers
$50.00/100mg
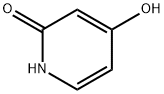
626-03-9
350 suppliers
$5.00/1g

237435-16-4
55 suppliers
$50.00/100mg
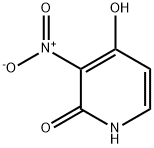
89282-12-2
254 suppliers
$8.00/5g

237435-16-4
55 suppliers
$50.00/100mg