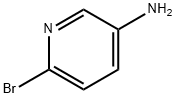
5-Amino-2-bromopyridine synthesis
- Product Name:5-Amino-2-bromopyridine
- CAS Number:13534-97-9
- Molecular formula:C5H5BrN2
- Molecular Weight:173.01
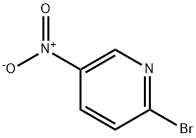
4487-59-6

13534-97-9
General procedure for the synthesis of 5-amino-2-bromopyridine from 2-bromo-5-nitropyridine: To a solution of 2-bromo-5-nitropyridine (2.03 g, 10 mmol) in ethanol (48 mL) was added sequentially iron powder (2.8 g, 50 mmol), concentrated hydrochloric acid (1.9 mL) and water (9.1 mL). The reaction mixture was stirred under reflux conditions for 5 hours. After completion of the reaction, it was cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure, the pH was adjusted to 7-8 with base and subsequently extracted with dichloromethane (DCM). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the reddish brown solid product 5-amino-2-bromopyridine (1.58 g, 91.3% yield).

4487-59-6
442 suppliers
$5.00/1g
462-08-8
476 suppliers
$14.00/25g

13534-97-9
437 suppliers
$6.00/1g
Yield:13534-97-9 88.5% ,462-08-8 11.5%
Reaction Conditions:
with hydrogen in water;toluene at 50; under 4500.45 Torr; for 5 h;Reagent/catalyst;
Steps:
1; 1; 2; 2; 3; 4; 5; 6; 7; 8; 9
Reactant: 2.5 mmol of 2-Bromo-5-nitrobenzene represented by the formula (C1-1) Pressure of hydrogen: 0.6 MPa Reaction temperature: 50° C. (Example 1, Example 2, Examples 4 to Example 9), 100° C. (Example 3, Comparative Example 1) Reaction time: 5 hours
References:
N.E. CHEMCAT CORPORATION;Suzuka, Hiroyasu;Mizusaki, Tomoteru;Nakaya, Yusuke;Wada, Yoshiyuki US2020/290967, 2020, A1 Location in patent:Paragraph 0097-0111

462-08-8
476 suppliers
$14.00/25g

13534-97-9
437 suppliers
$6.00/1g

4487-59-6
442 suppliers
$5.00/1g

13534-97-9
437 suppliers
$6.00/1g

4487-59-6
442 suppliers
$5.00/1g
7439-89-6
467 suppliers
$10.00/25g

13534-97-9
437 suppliers
$6.00/1g

462-08-8
476 suppliers
$14.00/25g

13534-97-9
437 suppliers
$6.00/1g
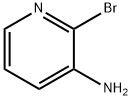
39856-58-1
307 suppliers
$10.00/1g
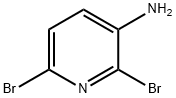
39856-57-0
299 suppliers
$10.00/1g