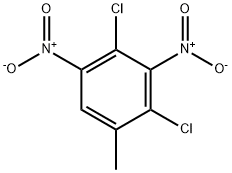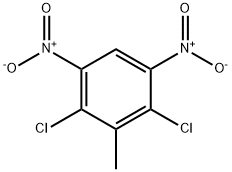
2,4-Dichloro-3-methyl-1,5-dinitrobenzene synthesis
- Product Name:2,4-Dichloro-3-methyl-1,5-dinitrobenzene
- CAS Number:51676-76-7
- Molecular formula:C7H4Cl2N2O4
- Molecular Weight:251.02
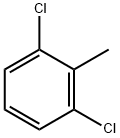
118-69-4
295 suppliers
$14.00/5g

51676-76-7
15 suppliers
inquiry
Yield:51676-76-7 93.5%
Reaction Conditions:
with sulfuric acid;sulfur trioxide;nitric acid;dinitrogen tetraoxide at 0 - 100;Product distribution / selectivity;Inert atmosphere;
Steps:
1
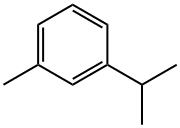
Example 1Preparation of DCDNTTo a 1 L 3-neck round bottom flask equipped with external ice cooling, mechanical stirrer, addition funnel, N2 inlet, and thermometer was added 174 g (2.76 mol) fuming nitric acid (d=1.54), followed by 350 g sulfuric acid and 659 g 30% oleum (2.0 molar equiv SO3) maintaining a temperature between 5 and 20 C. Subsequently, 199 g (1.23 mol) 1,3-dichlorotoluene (99% purity, Aldrich Chemical Company, Milwaukee, Wis., USA) was added over a time period of 3 h while maintaining a temperature between 0° C.-10° C. The ice bath was removed, and the reaction mixture was allowed to warm up to room temperature. It was then heated to 100° C. for about 2 h. To analyze the reaction mixture, a small sample of crude product was taken from the reaction vessel and poured into ice water. The crude product was extracted with methylene chloride. Analysis by GC indicated a reaction selectivity to 3,5-dinitro-2,6-dichlorotoluene of >97%. Subsequently, the reaction mixture was allowed to cool to room temperature over 2 h and then cooled to 5° C. over 30 min, after which it was filtered through a glass fritted funnel and washed with a little sulfuric acid followed by 200 mL H20. The wet cake contained about 20% water. After drying, 291 g of >99.5% pure DCDNT product (by 1H-NMR) was isolated (93.5% net yield).
References:
US2010/160685,2010,A1 Location in patent:Page/Page column 4
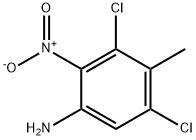
861559-26-4
0 suppliers
inquiry

51676-76-7
15 suppliers
inquiry

39182-94-0
1 suppliers
inquiry

51676-76-7
15 suppliers
inquiry

861294-58-8
0 suppliers
inquiry

51676-76-7
15 suppliers
inquiry