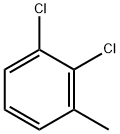
2,6-Dichlorotoluene synthesis
- Product Name:2,6-Dichlorotoluene
- CAS Number:118-69-4
- Molecular formula:C7H6Cl2
- Molecular Weight:161.03
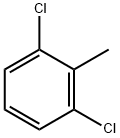
87-60-5
450 suppliers
$8.00/25g

118-69-4
296 suppliers
$14.00/5g
Yield:118-69-4 89%
Reaction Conditions:
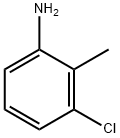
Stage #1: 3-chloro-2-methylbenzenaminewith potassium nitrite;potassium chloride in water at 8 - 90; for 0.666667 h;
Stage #2: with nickel nitrate;potassium chloride in water at 60; for 2.83333 h;Concentration;Temperature;Reagent/catalyst;
Steps:
3 Example 3
(I) In the case where a stirrer, a thermometer,The reaction vessel of the dropping funnel,Adding 500mL of potassium chloride solution with 50% mass, adding 500mL of aqueous solution,The solution temperature was maintained at 90 ° C and added in portions2-methyl-3-chloroaniline (2) 1.2 mol,After complete dissolution of the reactants,Maintaining the solution temperature to 9 ° C, dropping potassium nitrite 1.6moL dissolved in 200mL aqueous solution configured into a solution, the configuration process to maintain the solution temperature at 8 ,The configuration time is maintained at 40 minutes.The reaction end point was determined by potassium iodide test paper to form diazonium salt (3).(Ii) 0.5 mol of nickel nitrate and 0.5 mol of sodium nitrite were dissolved in 700 mL of an aqueous solution,500mL of 80% potassium chloride solution was added, the solution was heated to 60 ,A solution of nickel chloride,The solution of the diazonium salt (3) formed in step (i) was slowly added to the solution of nickel chloride,The stirring speed was maintained at 500 rpm, the duration of the addition was maintained at 50 min, and after 2 h,To be layered solution, the oil layer washed with hydrochloric acid 6 times,Washed 5 times with potassium chloride, 3 times with sodium carbonate,Finally, sodium bicarbonate solution was added to keep the pH of the solution at 7-8. The aqueous layer was separated and dehydrated with calcium oxide, and the fraction was collected at 180-192 ° C.To give 171.95 g of 2,6-dichlorotoluene in 89% yield.
References:
CN105541546,2016,A Location in patent:Paragraph 0008; 0018; 0019; 0020
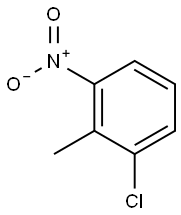
32768-54-0
267 suppliers
$9.00/25g

118-69-4
296 suppliers
$14.00/5g
83-42-1
278 suppliers
$9.00/5g

118-69-4
296 suppliers
$14.00/5g
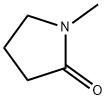
872-50-4
994 suppliers
$10.00/100ML
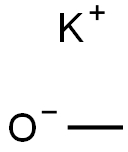
865-33-8
174 suppliers
$12.50/100G
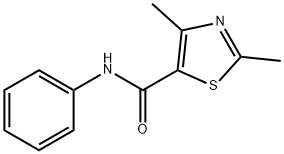
21452-18-6
12 suppliers
inquiry

118-69-4
296 suppliers
$14.00/5g
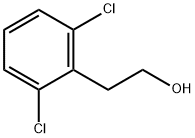
30595-79-0
143 suppliers
$8.00/250mg

118-69-4
296 suppliers
$14.00/5g
28469-92-3
99 suppliers
$40.00/500mg

289058-20-4
57 suppliers
inquiry