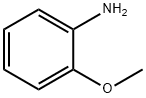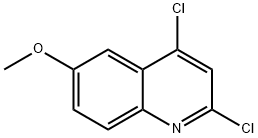
2,4-DICHLORO-6-METHOXYQUINOLINE synthesis
- Product Name:2,4-DICHLORO-6-METHOXYQUINOLINE
- CAS Number:70049-46-6
- Molecular formula:C10H7Cl2NO
- Molecular Weight:228.07
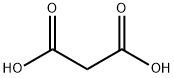
141-82-2
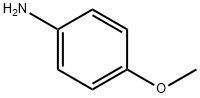
104-94-9

70049-46-6
1. In a 3-necked round-bottomed flask fitted with a condenser and a room temperature stirring bar, phosphorus (V) chloride oxide (40 mL, 1.5 M) and malonic acid (6.244 g, 60.00 mmol) were added. 2. p-Aminoanisole (9.236 g, 75.00 mmol) was added in small portions over 15 minutes under stirring conditions through an open neck portion of the round bottom flask. 3. The reaction mixture was heated to reflux and stirred continuously for 5 hours. 4. Upon completion of the reaction, the mixture was cooled to room temperature and then slowly poured into crushed ice (700 mL). 5. The pH of the resulting aqueous solution was adjusted to 10 with concentrated ammonium hydroxide (85 mL). 6. The aqueous phase was extracted using dichloromethane and the organic layers were combined. 7. The organic layer was dried over anhydrous magnesium sulfate and subsequently concentrated under reduced pressure. 8. The crude product was purified by column chromatography (eluent: 0-5% hexane solution of ethyl acetate) to afford 2,4-dichloro-6-methoxyquinoline as a pale yellow solid (4.7812 g, 35% yield). 9. Characterization of the product: melting point 170.5-171.5 °C; thin-layer chromatography (TLC) Rf = 0.27 (2% ethyl acetate/hexane); infrared spectroscopy (thin-film) νmax 3084, 3013, 2982, 1623, 1562, 1499 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J = 9.0 Hz, 1H), 7.45 (s, 1H), 7.5 (s, 1H). , 7.45 (s, 1H), 7.40 (dd, J = 9.0, 3.0 Hz, 1H), 7.36 (d, J = 3.0 Hz, 1H), 3.96 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 158.9, 147.0, 144.0, 142.6, 130.4, 126.3, 124.1, 122.0, 101.9, 55.7; high-resolution mass spectrometry data have been submitted.
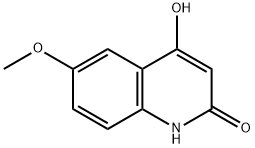
14300-45-9
62 suppliers
$16.00/100mg

70049-46-6
67 suppliers
$45.00/10mg
Yield:70049-46-6 84%
Reaction Conditions:
with trichlorophosphate for 8 h;Reflux;
References:
Enoua, Guy Crépin;Lahm, Günther;Uray, Georg;Stadlbauer, Wolfgang [Journal of Heterocyclic Chemistry,2014,vol. 51,# SUPPL. 1,p. E263-E275]

141-82-2
789 suppliers
$5.00/25g

104-94-9
497 suppliers
$9.00/1g

70049-46-6
67 suppliers
$45.00/10mg
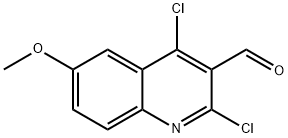
151772-24-6
14 suppliers
inquiry

70049-46-6
67 suppliers
$45.00/10mg

104-94-9
497 suppliers
$9.00/1g

70049-46-6
67 suppliers
$45.00/10mg