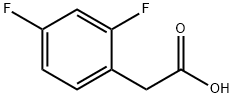
2,4-Difluorophenylacetic acid synthesis
- Product Name:2,4-Difluorophenylacetic acid
- CAS Number:81228-09-3
- Molecular formula:C8H6F2O2
- Molecular Weight:172.13
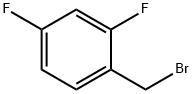
23915-07-3

81228-09-3
(a) 25.7 g of 2,4-difluorobenzyl bromide was dissolved in 100 mL of tetrahydrofuran and slowly added dropwise over 0.5 h to a stirred suspension containing 3.0 g of magnesium chips and 30 mL of tetrahydrofuran. After the dropwise addition was completed, stirring of the reaction mixture was continued for 10 minutes. Subsequently, a steady stream of carbon dioxide was continuously passed into the resulting Grignard reagent for 1 hour. After completion of the reaction, the mixture was evaporated to dryness and the residue was partitioned with ether and dilute hydrochloric acid. The ether phase was separated and extracted with 2 N sodium hydroxide solution. The basic extract was acidified with concentrated hydrochloric acid and extracted with ether to give 5.1 g (24% yield) of crude 2,4-difluorophenylacetic acid. The crude product was dissolved directly in 25 mL of tetrahydrofuran without further purification and added slowly dropwise to a stirred mixture of 2.0 g of lithium aluminum hydride suspended in 100 mL of tetrahydrofuran.
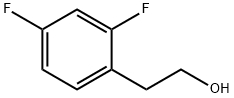
81228-02-6
14 suppliers
inquiry

81228-09-3
271 suppliers
$12.00/2 g
Yield:81228-09-3 146 g
Reaction Conditions:
with ozone in chlorobenzene at 0; for 1.5 h;
Steps:
The former oil was added to 180 g of chlorobenzene (1.62 mol, 200 ml), stirred and dissolved, and the system was cooled to 0 ° C, and slowly passed into O3.During this process, solids gradually precipitated. The reaction was completed in about 1.5 hours.The system was added with 150 ml of water, adjusted to pH 11 with NaOH, and the organic layer was separated.The aqueous layer was back-extracted with 100 ml x 3 dichloromethane.After separating the organic layer, the aqueous layer was adjusted to pH 1 with concentrated hydrochloric acid.A large amount of crystals precipitated. Filter, wash the filter cake with ice water, dry,A white powder of 146 g of 2,4-difluorophenylacetic acid was obtained.The yield was 85% based on m-difluorobenzene.
References:
Zhejiang Shaxing Technology Co., Ltd.;Huang Xiaoting CN108752218, 2018, A Location in patent:Paragraph 0026

23915-07-3
257 suppliers
$9.00/5g

81228-09-3
271 suppliers
$12.00/2 g
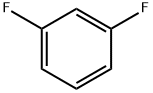
372-18-9
424 suppliers
$9.00/1g

81228-09-3
271 suppliers
$12.00/2 g