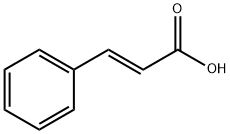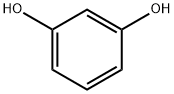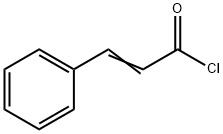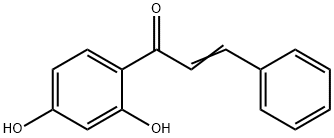
2',4'-DIHYDROXYCHALCONE synthesis
- Product Name:2',4'-DIHYDROXYCHALCONE
- CAS Number:1776-30-3
- Molecular formula:C15H12O3
- Molecular Weight:240.25
Yield:1776-30-3 64%
Reaction Conditions:
with sodium hydroxide in ethanol at 20;Claisen-Schmidt condensation;
Steps:
4.2. General procedures for synthesis of chalcones
General procedure: For synthesis of chalcones we have used 2 different methods. Chalcones 1-30 were synthesized by method A and chalcones 31-44 were synthesized by method B. For Method B we used LiOHinstead of usual base NaOH for Claisen-Schmidt synthesis and the reaction was carried out in an ultrasonic bath. The general reaction scheme is depicted in Scheme 1.Method A: 20% NaOH (5 ml) was added dropwise to a previously cooled mixture of 5 mmol of selected acetophenone and 5 mmol of selected benzaldehyde in 25 ml ethanol under vigorous stirring. The mixture was stirred at room temperature for 24-72 h. After completion of the reaction as indicated by TLC, the mixture was poured onto crushed ice and acidified with dilute HCl. Precipitated product was filtered by suction and washed to neutral. The solid was recrystallised from dilute ethanol to get crystalline chalcone.Method B: To a mixture of 5 mmol of selected acetophenone and 5 mmol of selected benzaldehyde in 50 ml round bottom flask, 20 ml methanol and 35 mmol of LiOH was added. The reaction mixture was kept in an ultrasonic bath for 1-5 h, until reaction completion was indicated by TLC. The reaction was worked up as described for method A.
References:
Juvale, Kapil;Pape, Veronika F.S.;Wiese, Michael [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 1,p. 346 - 355] Location in patent:experimental part
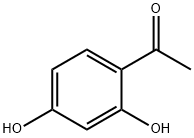
89-84-9
564 suppliers
$6.00/10g

1776-30-3
58 suppliers
inquiry
![1-[2-hydroxy-4-(methoxymethoxy)phenyl]ethanone](/CAS/20180703/GIF/65490-08-6.gif)
65490-08-6
5 suppliers
inquiry

1776-30-3
58 suppliers
inquiry