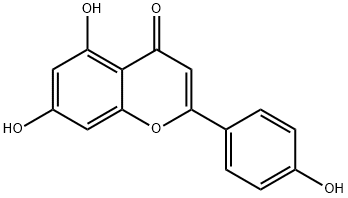
Apigenin synthesis
- Product Name:Apigenin
- CAS Number:520-36-5
- Molecular formula:C15H10O5
- Molecular Weight:270.24
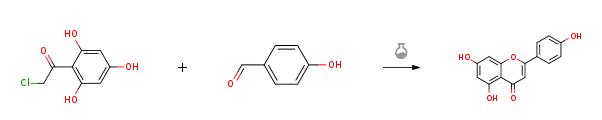
5631-70-9
120 suppliers
$119.00/5mg

520-36-5
662 suppliers
$5.00/100mg
Yield:520-36-5 90%
Reaction Conditions:
with pyridine hydrochloride at 180; for 6 h;Inert atmosphere;
Steps:
Apigenin (1):
Compound 5 (1.4 g, 0.005 mol) and excess pyridinehydrochloride (5.0 g, 0.04 mol) were heated at 180 °C for 6 h under aN2 atmosphere. The mixture was cooled to room temperature and H2O(100 mL) was added. The mixture was stirred for another 30 min andcooled to below 5 °C for several hours. The precipitate was filteredoff, washed with cold ethanol and recrystallised from absoluteethanol to give compound 1 as yellow crystals (1.2 g, yield 90%);
References:
Wang, Qian;Cui, Wei;Liu, Man;Zhang, Ji;Liao, Rong-Qiang;Liao, Xia-Li;Yang, Jian [Journal of Chemical Research,2015,vol. 39,# 2,p. 67 - 69]
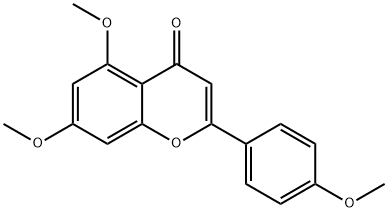
480-41-1
441 suppliers
$5.00/5g

520-36-5
662 suppliers
$5.00/100mg
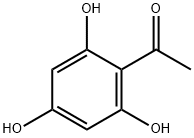
480-66-0
359 suppliers
$10.00/1g
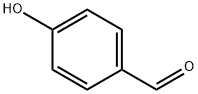
123-08-0
952 suppliers
$5.00/10g

520-36-5
662 suppliers
$5.00/100mg
![1,3,5-Benzenetriol, 2-[3-(4-hydroxyphenyl)-5-isoxazolyl]-](/CAS/20210305/GIF/141993-07-9.gif)
141993-07-9
0 suppliers
inquiry

520-36-5
662 suppliers
$5.00/100mg
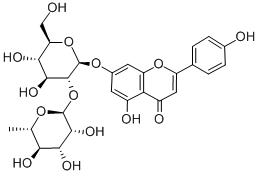
17306-46-6
212 suppliers
$50.00/5mg

520-36-5
662 suppliers
$5.00/100mg