2-(4-FLUOROPHENYL)IMIDAZO[1,2-A]PYRIMIDINE synthesis
- Product Name:2-(4-FLUOROPHENYL)IMIDAZO[1,2-A]PYRIMIDINE
- CAS Number:397-53-5
- Molecular formula:C12H8FN3
- Molecular Weight:213.21
Yield:397-53-5 85%
Reaction Conditions:
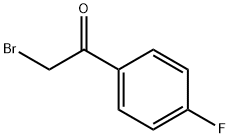
Stage #1: 1-(4-Fluorophenyl)ethanonewith [Hydroxy(tosyloxy)iodo]benzene;N-butylpyridinium tetrafluoroborateHeating;
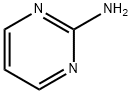
Stage #2: 2-aminopyrimidinewith sodium carbonate;N-butylpyridinium tetrafluoroborate at 20;
References:
Xie, Yuan-Yuan [Synthetic Communications,2005,vol. 35,# 13,p. 1741 - 1746]
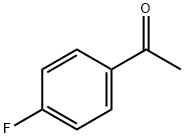
403-42-9
555 suppliers
$5.00/10g
![2-(4-FLUOROPHENYL)IMIDAZO[1,2-A]PYRIMIDINE](/StructureFile/ChemBookStructure4/GIF/CB5329960.gif)
397-53-5
13 suppliers
$45.00/10mg