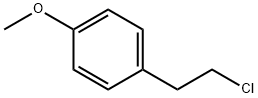
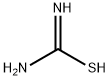
2-(4-methoxyphenyl)ethane-1-thiol synthesis
- Product Name:2-(4-methoxyphenyl)ethane-1-thiol
- CAS Number:63659-59-6
- Molecular formula:C9H12OS
- Molecular Weight:168.26
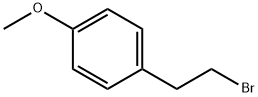
14425-64-0
66 suppliers
$22.00/250mg

63659-59-6
3 suppliers
$809.00/1g
Yield:63659-59-6 88%
Reaction Conditions:
with sodium hydroxide;thiourea in ethanol;
Steps:
XIV Preparation of the Starting Compound: 4-(2-Cyclobutylmethylthio-ethyl)-phenol
28 g (0.130 mol) of 2-(p-methoxyphenyl)-ethyl bromide, 9.90 g (0.130 mol) of thiourea and 250 ml of ethanol are introduced into an Erlenmeyer flask placed under nitrogen and equipped with a stirrer and a reflux condenser. The mixture is heated under reflux for about 2 hours. It is then allowed to cool and 130 ml of 2 N sodium hydroxide solution are added. This mixture is heated under reflux for 2 hours. The ethanol is evaporated and after cooling the residue is acidified with concentrated hydrochloric acid and extracted with ether. The ether phase is washed with water, with an aqueous bicarbonate solution and again with water and is dried over magnesium sulphate, and the ether is evaporated. 21.4 g of oil are obtained; this oil, after distillation under 0.1 mm Hg at a temperature of 115° C., gives 19.2 g of 2-(p-methoxyphenyl)-ethanethiol. Yield: 88%. Analysis: Calculated %: C, 64.25; H, 7.19; S, 19.06. Found %: C, 64.55; H, 7.43; S, 18.96; C, 64.33; H, 7.40; S, 19.05.
References:
US4252984,1981,A

14425-64-0
66 suppliers
$22.00/250mg
17356-08-0
2 suppliers
inquiry

63659-59-6
3 suppliers
$809.00/1g
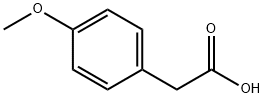
104-01-8
542 suppliers
$6.00/10g

63659-59-6
3 suppliers
$809.00/1g
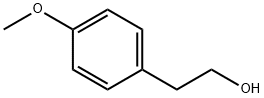
702-23-8
164 suppliers
$9.00/5g

63659-59-6
3 suppliers
$809.00/1g