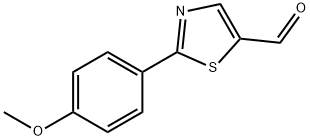
2-(4-METHOXYPHENYL)THIAZOLE-5-CARBALDEHYDE synthesis
- Product Name:2-(4-METHOXYPHENYL)THIAZOLE-5-CARBALDEHYDE
- CAS Number:914348-82-6
- Molecular formula:C11H9NO2S
- Molecular Weight:219.26

27088-84-2

68-12-2

914348-82-6
1. Palladium acetate (0.82 g, 3.66 mmol), triphenylphosphine (4.80 g, 18.29 mmol) and 2 M aqueous sodium carbonate (183.0 mL, 366.0 mmol) were sequentially added under nitrogen protection to a DMF (400 mL) solution containing 2-bromothiazole (20.00 g, 121.94 mmol) and 4-methoxyphenylboronic acid (25.94 g, 170.71 mmol). 170.71 mmol) in a solution of DMF (400 mL). The reaction mixture was stirred at 100 °C for 3 hours. After completion of the reaction, water was added to the reaction solution and extracted with a solvent mixture of ethyl acetate and toluene (1:1, v/v). The organic phases were combined, washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate) to give a yellow oily thiazole derivative (20.40 g, 88% yield). 2. n-Butyllithium (2.64 M hexane solution, 52.5 mL, 138.67 mmol) was slowly added dropwise to a solution of THF (500 mL) of the above thiazole derivative (20.40 g, 106.67 mmol) at -78 °C and under nitrogen protection. After the dropwise addition, stirring was continued at -78 °C for 2 hours. Subsequently, DMF (16.4 mL, 213.33 mmol) was added slowly dropwise and the reaction system was slowly warmed to 0 °C and stirring was continued for 2 hours. Upon completion of the reaction, the reaction was quenched by dropwise addition of acetic acid (7.9 mL, 138.67 mmol) and the solvent was removed by distillation under reduced pressure. The residue was dissolved in ethyl acetate and partitioned by adding water. The aqueous phase was extracted with ethyl acetate, the organic phases were combined, washed with water and saturated brine sequentially, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was recrystallized with diisopropyl ether to obtain 2-(4-methoxyphenyl)thiazole-5-carbaldehyde (18.31 g, 78% yield) as yellow powder.
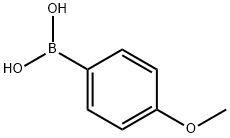
5720-07-0
468 suppliers
$6.00/5g

914348-82-6
55 suppliers
$38.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: sodium carbonate / triphenylphosphine; palladium diacetate / N,N-dimethyl-formamide; water / 3 h / 100 °C
2.1: n-butyllithium / hexane; tetrahydrofuran / -78 °C
2.2: 0 °C
References:
Daiichi Sankyo Company, Limited EP2308838, 2011, A1
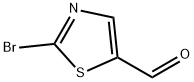
464192-28-7
161 suppliers
$12.00/250mg

5720-07-0
468 suppliers
$6.00/5g

914348-82-6
55 suppliers
$38.00/100mg