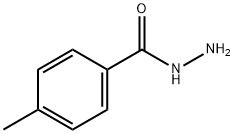
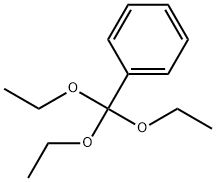
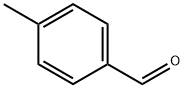
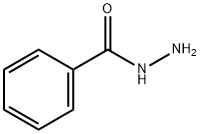
2-(4-METHYLPHENYL)-5-PHENYL-1,3,4-OXADIAZOLE synthesis
- Product Name:2-(4-METHYLPHENYL)-5-PHENYL-1,3,4-OXADIAZOLE
- CAS Number:1874-47-1
- Molecular formula:C15H12N2O
- Molecular Weight:236.27
Yield:1874-47-1 96%
Reaction Conditions:
with ammonium chloride in ethanol; for 2.5 h;Reflux;
Steps:
Representative procedure for the 1,3,4-oxadiazoles synthesis:
General procedure: To asolution of hydrazide (0.73 mmol) in 10 mL of anhydrous ethanol, added triethyl orthoester (0.81 mmol) and ammonium chloride (0.219 mmol). The solution was stirred and heated to reflux until it complete (0.5-18 h). The reaction mass was cooled to room temperature and concentrated under vacuum. The crude product was made into slurry in a mixture of hexanes and ether (3:1), filtered,washed with deionized water or purified by column chromatography.
References:
Gnanasekaran, Krishna Kumar;Nammalwar, Baskar;Murie, Maeghan;Bunce, Richard A. [Tetrahedron Letters,2014,vol. 55,# 50,p. 6776 - 6778] Location in patent:supporting information